Combined Inhibition of AKT and KIT Restores Expression of Programmed Cell Death 4 (PDCD4) in Gastrointestinal Stromal Tumor
- PMID: 34359600
- PMCID: PMC8345102
- DOI: 10.3390/cancers13153699
Combined Inhibition of AKT and KIT Restores Expression of Programmed Cell Death 4 (PDCD4) in Gastrointestinal Stromal Tumor
Abstract
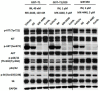
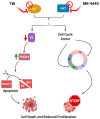
The majority of gastrointestinal stromal tumor (GIST) patients develop resistance to the first-line KIT inhibitor, imatinib mesylate (IM), through acquisition of secondary mutations in KIT or bypass signaling pathway activation. In addition to KIT, AKT is a relevant target for inhibition, since the PI3K/AKT pathway is crucial for IM-resistant GIST survival. We evaluated the activity of a novel pan-AKT inhibitor, MK-4440 (formerly ARQ 751), as monotherapy and in combination with IM in GIST cell lines and preclinical models with varying IM sensitivities. Dual inhibition of KIT and AKT demonstrated synergistic effects in IM-sensitive and -resistant GIST cell lines. Proteomic analyses revealed upregulation of the tumor suppressor, PDCD4, in combination treated cells. Enhanced PDCD4 expression correlated to increased cell death. In vivo studies revealed superior efficacy of MK-4440/IM combination in an IM-sensitive preclinical model of GIST compared with either single agent. The combination demonstrated limited efficacy in two IM-resistant models, including a GIST patient-derived xenograft model possessing an exon 9 KIT mutation. These studies provide strong rationale for further use of AKT inhibition in combination with IM in primary GIST; however, alternative agents will need to be tested in combination with AKT inhibition in the resistant setting.
Keywords: AKT; GIST; PDCD4; gastrointestinal stromal tumor; imatinib mesylate.
Conflict of interest statement
L.R. and M.v.M. have received research support from ArQule, Inc. Y.Y. and B.S. were previously employed by ArQule, Inc. and own stock in ArQule, Inc. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





Similar articles
-
Unraveling the Mechanisms of Sensitivity to Anti-FGF Therapies in Imatinib-Resistant Gastrointestinal Stromal Tumors (GIST) Lacking Secondary KIT Mutations.Cancers (Basel). 2023 Nov 9;15(22):5354. doi: 10.3390/cancers15225354. Cancers (Basel). 2023. PMID: 38001614 Free PMC article.
-
Preclinical Activity of PI3K Inhibitor Copanlisib in Gastrointestinal Stromal Tumor.Mol Cancer Ther. 2020 Jun;19(6):1289-1297. doi: 10.1158/1535-7163.MCT-19-1069. Epub 2020 May 5. Mol Cancer Ther. 2020. PMID: 32371592
-
Fibroblast Growth Factor 2 (FGF2) Activates Vascular Endothelial Growth Factor (VEGF) Signaling in Gastrointestinal Stromal Tumors (GIST): An Autocrine Mechanism Contributing to Imatinib Mesylate (IM) Resistance.Cancers (Basel). 2024 Sep 7;16(17):3103. doi: 10.3390/cancers16173103. Cancers (Basel). 2024. PMID: 39272961 Free PMC article.
-
Inhibition of FGF2-Mediated Signaling in GIST-Promising Approach for Overcoming Resistance to Imatinib.Cancers (Basel). 2020 Jun 24;12(6):1674. doi: 10.3390/cancers12061674. Cancers (Basel). 2020. PMID: 32599808 Free PMC article. Review.
-
Imatinib mesylate: in the treatment of gastrointestinal stromal tumours.Drugs. 2003;63(5):513-22; discussion 523-4. doi: 10.2165/00003495-200363050-00005. Drugs. 2003. PMID: 12600228 Review.
Cited by
-
SHP2 inhibition and adjuvant therapy synergistically target KIT-mutant GISTs via ERK1/2-regulated GSK3β/cyclin D1 pathway.Clin Transl Med. 2025 Feb;15(2):e70231. doi: 10.1002/ctm2.70231. Clin Transl Med. 2025. PMID: 39981588 Free PMC article.
-
Current Drug Resistance Mechanisms and Treatment Options in Gastrointestinal Stromal Tumors: Summary and Update.Curr Treat Options Oncol. 2024 Nov;25(11):1390-1405. doi: 10.1007/s11864-024-01272-7. Epub 2024 Oct 23. Curr Treat Options Oncol. 2024. PMID: 39441520 Free PMC article. Review.
References
-
- Belinsky M.G., Rink L., Flieder D.B., Jahromi M.S., Schiffman J.D., Godwin A.K., von Mehren M. Overexpression of insulin-like growth factor 1 receptor and frequent mutational inactivation of SDHA in wild-type SDHB-negative gastrointestinal stromal tumors. Genes Chromosom. Cancer. 2013;52:214–224. doi: 10.1002/gcc.22023. - DOI - PMC - PubMed
-
- Joensuu H., Vehtari A., Riihimäki J., Nishida T., Steigen S.E., Brabec P., Plank L., Nilsson B., Cirilli C., Braconi C., et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–274. doi: 10.1016/S1470-2045(11)70299-6. - DOI - PubMed
-
- Demetri G.D., von Mehren M., Blanke C.D., Van den Abbeele M.D., Eisenberg B., Roberts P.J., Heinrich M.C., Tuveson D.A., Singer S., Janicek M., et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

