Association of the Lung Immune Prognostic Index with Immunotherapy Outcomes in Mismatch Repair Deficient Tumors
- PMID: 34359675
- PMCID: PMC8345164
- DOI: 10.3390/cancers13153776
Association of the Lung Immune Prognostic Index with Immunotherapy Outcomes in Mismatch Repair Deficient Tumors
Abstract
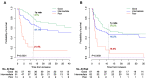
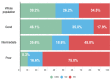
Background: MSI-H/dMMR is considered the first predictive marker of efficacy for immune checkpoint inhibitors (ICIs). However, around 39% of cases are refractory and additional biomarkers are needed. We explored the prognostic value of pretreatment LIPI in MSI-H/dMMR patients treated with ICIs, including identification of fast-progressors. Methods: A multicenter retrospective study of patients with metastatic MSI-H/dMMR tumors treated with ICIs between April 2014 and May 2019 was performed. LIPI was calculated based on dNLR > 3 and LDH > upper limit of normal. LIPI groups were good (zero factors), intermediate (one factor) and poor (two factors). The primary endpoint was overall survival (OS), including the fast-progressor rate (OS < 3 months). Results: A total of 151 patients were analyzed, mainly female (59%), with median age 64 years, performance status (PS) 0 (42%), and sporadic dMMR status (68%). ICIs were administered as first or second-line for 59%. The most frequent tumor types were gastrointestinal (66%) and gynecologic (22%). LIPI groups were good (47%), intermediate (43%), and poor (10%). The median follow-up was 32 months. One-year OS rates were 81.0%, 67.1%, and 21.4% for good, intermediate, and poor-risk groups (p < 0.0001). After adjustment for tumor site, metastatic sites and PS, LIPI remained independently associated with OS (HR, poor-LIPI: 3.50, 95%CI: 1.46-8.40, p = 0.02. Overall, the fast-progressor rate was 16.0%, and 35.7% with poor-LIPI vs. 7.5% in the good-LIPI group (p = 0.02). Conclusions: LIPI identifies dMMR patients who do not benefit from ICI treatment, particularly fast-progressors. LIPI should be included as a stratification factor for future trials.
Keywords: LDH; LIPI; MSI-H; dMMR; dNLR; immune checkpoint inhibitors; immunotherapy.
Conflict of interest statement
E. Auclin: Travel/Accommodation/Expenses: Mundipharma; Honoraria (self): Sanofi Genzymes. J. Taieb: Honoraria (self): Merck, Roche, Amgen, Lilly, Sanofi, Samsung, MSD, Servier, Celgene, Pierre Fabre; Advisory/Consultancy: Roche, Merck KGaA, Amgen, Lilly, MSD, Servier, Pierre Fabre, Sanofi, Samsung; Speaker Bureau/Expert testimony: Servier, Amgen, Roche, Sanofi, Merck, Lilly, Pierre Fabre. B. Besse: sponsored research at Gustave Roussy Cancer Center 4D Pharma, Abbvie, Amgen, Aptitude Health, AstraZeneca, BeiGene, Blueprint Medicines, BMS, Boehringer Ingelheim, Celgene, Cergentis, Cristal Therapeutics, Daiichi-Sankyo, Eli Lilly, GSK, Inivata, Janssen, Onxeo, OSE immunotherapeutics, Pfizer, Roche-Genentech, Sanofi, Takeda, Tolero Pharmaceuticals. C. Massard: Advisory/Consultancy: Amgen, Astellas, AstraZeneca, Bayer, BeiGene, BMS, Celgene, Debiopharm, Genentech, Ipsen, Janssen, Lilly, MedImmune, MSD, Novartis, Pfizer, Roche, Sanofi, Orion. L. Mezquita: Research grant/Funding (self): Bristol Myers Squibb, Boehringer Ingelheim, Amgen, Stilla, Inivata; Advisory/Consultancy: Roche Diagnostics, Takeda; Honoraria (self): Bristol Myers Squibb, Tecnofarma, Roche; Travel/Accommodation/Expenses: Roche; Non-remunerated activity/ies: AstraZeneca. A. Hollebecque: Advisory/Consultancy: Gritstone Oncology, Eisai Co., Ltd., Amgen, Servier and Merck Serono. All other authors have declared no conflicts of interest.
Figures
References
-
- Luchini C., Bibeau F., Ligtenberg M., Singh N., Nottegar A., Bosse T., Miller R., Riaz N., Douillard J.-Y., Andre F., et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019;30:1232–1243. doi: 10.1093/annonc/mdz116. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

