Risk Factors for Liver Decompensation and HCC in HCV-Cirrhotic Patients after DAAs: A Multicenter Prospective Study
- PMID: 34359711
- PMCID: PMC8345116
- DOI: 10.3390/cancers13153810
Risk Factors for Liver Decompensation and HCC in HCV-Cirrhotic Patients after DAAs: A Multicenter Prospective Study
Abstract
Background: Prospective studies on predictors of liver-related events in cirrhotic subjects achieving SVR after DAAs are lacking.
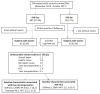
Methods: We prospectively enrolled HCV cirrhotic patients in four Italian centers between November 2015 and October 2017. SVR and no-SVR cases were compared according to the presence or absence of liver-related events during a 24-month follow-up. Independent predictors of liver-related events were evaluated by Cox regression analysis.
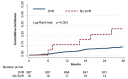
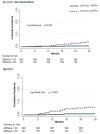
Results: A total of 706 subjects started DAAs therapy. SVR was confirmed in 687 (97.3%). A total of 61 subjects (8.9%) in the SVR group and 5 (26.3%) in the no-SVR group had liver-related events (p < 0.03). The incidence rate x 100 p/y was 1.6 for HCC, 1.7 for any liver decompensation, and 0.5 for hepatic death. Baseline liver stiffness (LSM) ≥ 20 kPa (HR 4.0; 95% CI 1.1-14.1) and genotype different from 1 (HR 7.5; 95% CI 2.1-27.3) were both independent predictors of liver decompensation. Baseline LSM > 20 KPa (HR 7.2; 95% CI 1.9-26.7) was the sole independent predictor of HCC. A decrease in liver stiffness (Delta LSM) by at least 20% at the end of follow-up was not associated with a decreased risk of liver-related events.
Conclusion: Baseline LSM ≥ 20 kPa identifies HCV cirrhotic subjects at higher risk of liver-related events after SVR.
Keywords: DAAs; HCC; HCV; cirrhosis; liver decompensation; liver stiffness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

