Survival of Patients with Epidermal Growth Factor Receptor-Mutated Metastatic Non-Small Cell Lung Cancer Treated beyond the Second Line in the Tyrosine Kinase Inhibitor Era
- PMID: 34359788
- PMCID: PMC8345514
- DOI: 10.3390/cancers13153887
Survival of Patients with Epidermal Growth Factor Receptor-Mutated Metastatic Non-Small Cell Lung Cancer Treated beyond the Second Line in the Tyrosine Kinase Inhibitor Era
Abstract
Background: The identification of activating mutations in specific genes led to the development of targeted therapies for NSCLC. TKI directed against EGFR-mutations were the first to prove their major efficacy. Medical associations recommend their use as first and second-line metastatic treatments in EGFR-mutated patients. Our objective was to analyze the survival of EGFR-mutated patients treated beyond the second line of treatment.
Methods: We performed a longitudinal, retrospective and analytical study at APHP (Assistance Publique Hopitaux de Paris) Saint Louis, Paris, France, from 1 January 2010 to 31 December 2020 (11 years), on EGFR-mutated patients with metastatic NSCLC which received TKI or chemotherapy (CT) in third-line.
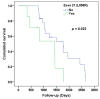
Results: Out of about 107 EGFR-mutated patients, 31 patients who benefited from TKI or CT in the third line of treatment were retained for this study. The mean age was 60.03 ± 11.93 years and the sex ratio male/female was 0.24. Mutations of exon 19, 21 and 20 were found in 21 (67.7%), 7 (22.6%) and 7 (22.6%) patients, respectively. Third-line treatment was CT for 16 patients (51.6%) and TKI for the 15 remaining patients (48.4%). Osimertinib was the most used TKI in third-line (n = 10/15; 66.67%). The median duration of third-line treatment was 5.37 months (range 0.53-37.6) and the median follow-up duration was 40.83 months (range 11.33-88.57). There was a significant difference in PFS between patients treated with TKI and CT in third-line (p = 0.028). For patients treated with CT in second-line, there was a significant difference of PFS (p < 0.001) and OS (p = 0.014) in favor of the use of TKI in third-line.
Conclusions: For patients receiving CT in second-line, TKI appears to be a better alternative in third-line compared to CT. Osimertinib may be used in third line treatment if not used before.
Keywords: EGFR-genes; TKI; drug therapy; lung cancer; metastasis; survival analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hanna N.H., Schneider B.J., Temin S., Baker S., Brahmer J., Ellis P.M., Gaspar L.E., Haddad R.Y., Hesketh P.J., Jain D., et al. Therapy for Stage IV Non–Small-Cell Lung Cancer Without Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J. Clin. Oncol. 2020;38:1608–1632. doi: 10.1200/JCO.19.03022. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

