Plasma BRAF Mutation Detection for the Diagnostic and Monitoring Trajectory of Patients with LDH-High Stage IV Melanoma
- PMID: 34359813
- PMCID: PMC8345527
- DOI: 10.3390/cancers13153913
Plasma BRAF Mutation Detection for the Diagnostic and Monitoring Trajectory of Patients with LDH-High Stage IV Melanoma
Abstract
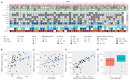
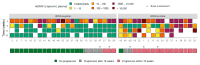
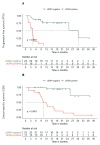
For patients with newly diagnosed metastatic melanoma, rapid BRAF mutation (mBRAF) assessment is vital to promptly initiate systemic therapy. Additionally, blood-based biomarkers are desired to monitor and predict treatment response. Circulating tumor DNA (ctDNA) has shown great promise for minimally invasive mBRAF assessment and treatment monitoring, but validation studies are needed. This prospective study utilized longitudinal plasma samples at regular timepoints (0, 6, 12, 18 weeks) to address the clinical validity of ctDNA measurements in stage IV melanoma patients with elevated serum lactate dehydrogenase (LDH > 250U/L) starting first-line systemic treatment. Using droplet digital PCR, the plasma mBRAF abundance was assessed in 53 patients with a BRAFV600 tissue mutation. Plasma mBRAF was detected in 50/51 patients at baseline (98% sensitivity; median fraction abundance of 19.5%) and 0/17 controls (100% specificity). Patients in whom plasma mBRAF became undetectable during the first 12-18 weeks of treatment had a longer progression-free survival (30.2 vs. 4.0 months; p < 0.001) and cancer-specific survival (not reached vs. 10.2 months; p < 0.001) compared to patients with detectable mBRAF. The ctDNA dynamics outperformed LDH and S100 dynamics. These results confirm the clinical validity of ctDNA measurements as a minimally invasive biomarker for the diagnostic and monitoring trajectory of patients with LDH-high stage IV melanoma.
Keywords: biomarker; circulating tumor DNA (ctDNA), BRAF; lactate dehydrogenase (LDH), S100; melanoma.
Conflict of interest statement
Rutger Koornstra reports receiving speaker fees from BMS, MSD, and Roche; he takes part in the advisory boards of BMS, MSD, Novartis, Roche, and received research funding from Roche and BMS. Jan Willem de Groot takes part in the advisory boards of Bristol-Myers Squibb, Roch, Pierre Fabre, Servier, MSD, and Novartis. Marye Boers-Sonderen takes part in the advisory boards of Pierre-Fabre, Merck, and Pfizer. Winald Gerritsen reports receiving research funding from Astellas, Amgen, Bayer, Janssen-Cilaq, and Sanofi; speaker fees from Astellas Bayer, Merck Sharp & Dohme (MSD), and the European Society for Medical Oncology, and he takes part in the advisory boards of Amgen, Bayer, Bristol-Myers Squibb, Curevac, Dendreon, IQVIA, Janssen-Cilag, MSD, Morphosys, and Sanofi. Marjolijn Ligtenberg reports receiving diagnostic innovation grants from AstraZeneca and Bristol-Myers Squibb; speaker fees from Roche, Merck Sharp & Dohme, and AstraZeneca; and she takes part in the advisory boards of Lilly, Janssen Pharmaceuticals, Merck Sharp & Dohme, AstraZeneca, Bristol-Myers Squibb, Bayer, and Novartis. Niven Mehra reports receiving research funding from Astellas, Janssen, Pfizer, Roche, and Sanofi; travel support of Astellas and MSD, and takes part in the advisory boards of Roche, MSD, BMS, Bayer, Astellas, and Janssen.
Figures
References
-
- Long G.V., Flaherty K.T., Stroyakovskiy D., Gogas H., Levchenko E., de Braud F., Larkin J., Garbe C., Jouary T., Hauschild A., et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann. Oncol. 2017;28:1631–1639. doi: 10.1093/annonc/mdx176. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

