NNRTI and Liver Damage: Evidence of Their Association and the Mechanisms Involved
- PMID: 34359857
- PMCID: PMC8303744
- DOI: 10.3390/cells10071687
NNRTI and Liver Damage: Evidence of Their Association and the Mechanisms Involved
Abstract
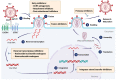
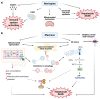
Due to the improved effectiveness and safety of combined antiretroviral therapy, human immunodeficiency virus (HIV) infection has become a manageable, chronic condition rather than a mortal disease. However, HIV patients are at increased risk of experiencing non-AIDS-defining illnesses, with liver-related injury standing out as one of the leading causes of death among these patients. In addition to more HIV-specific processes, such as antiretroviral drug-related toxicity and direct injury to the liver by the virus itself, its pathogenesis is related to conditions that are also common in the general population, such as alcoholic and non-alcoholic fatty liver disease, viral hepatitis, and ageing. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are essential components of combined anti-HIV treatment due to their unique antiviral activity, high specificity, and acceptable toxicity. While first-generation NNRTIs (nevirapine and efavirenz) have been related largely to liver toxicity, those belonging to the second generation (etravirine, rilpivirine and doravirine) seem to be generally safe for the liver. Indeed, there is preclinical evidence of rilpivirine being hepatoprotective in different models of liver injury, independently of the presence of HIV. The present study aims to review the mechanisms by which currently available anti-HIV drugs belonging to the NNRTI family may participate in the development of liver disease.
Keywords: DILI; HIV; antiretroviral drugs; cART; hepatotoxicity; liver.
Conflict of interest statement
J.V.E. has received funds for speaking at symposia organized by Abbvie Farmacéutica S.L.U., Astra Zeneca and Gilead Sciences. Other authors: none to declare.
Figures

References
-
- Smith C.J., Ryom L., Weber R., Morlat P., Pradier C., Reiss P., Kowalska J.D., De Wit S., Law M., El Sadr W., et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): A multicohort collaboration. Lancet. 2014;384:241–248. doi: 10.1016/S0140-6736(14)60604-8. - DOI - PubMed
-
- Palella F.J., Jr., Baker R.K., Moorman A.C., Chmiel J.S., Wood K.C., Brooks J.T., Holmberg S.D. HIV Outpatient Study Investigators. Mortality in the highly active antiretroviral therapy era: Changing causes of death and disease in the HIV outpatient study. J. Acquir. Immune Defic. Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

