Tuning IgE: IgE-Associating Molecules and Their Effects on IgE-Dependent Mast Cell Reactions
- PMID: 34359869
- PMCID: PMC8305778
- DOI: 10.3390/cells10071697
Tuning IgE: IgE-Associating Molecules and Their Effects on IgE-Dependent Mast Cell Reactions
Abstract
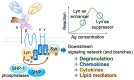
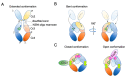
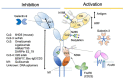
The recent emergence of anti-immunoglobulin E (IgE) drugs and their candidates for humans has endorsed the significance of IgE-dependent pathways in allergic disorders. IgE is distributed locally in the tissues or systemically to confer a sensory mechanism in a domain of adaptive immunity to the otherwise innate type of effector cells, namely, mast cells and basophils. Bound on the high-affinity IgE receptor FcεRI, IgE enables fast memory responses against revisiting threats of venoms, parasites, and bacteria. However, the dysregulation of IgE-dependent reactions leads to potentially life-threatening allergic diseases, such as asthma and anaphylaxis. Therefore, reactivity of the IgE sensor is fine-tuned by various IgE-associating molecules. In this review, we discuss the mechanistic basis for how IgE-dependent mast cell activation is regulated by the IgE-associating molecules, including the newly developed therapeutic candidates.
Keywords: CD23; FcεRI; IgE; basophils; glycosylation; histamine-releasing factor (HRF); mast cells; omalizumab; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Martin R.K., Damle S.R., Valentine Y.A., Zellner M.P., James B.N., Lownik J.C., Luker A.J., Davis E.H., DeMeules M.M., Khandjian L.M., et al. B1 Cell IgE Impedes Mast Cell-Mediated Enhancement of Parasite Expulsion through B2 IgE Blockade. Cell Rep. 2018;22:1824–1834. doi: 10.1016/j.celrep.2018.01.048. - DOI - PMC - PubMed
-
- Starkl P., Watzenboeck M.L., Popov L.M., Zahalka S., Hladik A., Lakovits K., Radhouani M., Haschemi A., Marichal T., Reber L.L., et al. IgE Effector Mechanisms, in Concert with Mast Cells, Contribute to Acquired Host Defense against Staphylococcus aureus. Immunity. 2020;53:1333. doi: 10.1016/j.immuni.2020.11.012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

