Viscoelastic Properties in Cancer: From Cells to Spheroids
- PMID: 34359874
- PMCID: PMC8304080
- DOI: 10.3390/cells10071704
Viscoelastic Properties in Cancer: From Cells to Spheroids
Abstract
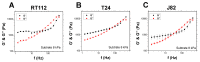
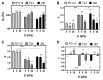
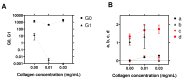
AFM-based rheology methods enable the investigation of the viscoelastic properties of cancer cells. Such properties are known to be essential for cell functions, especially for malignant cells. Here, the relevance of the force modulation method was investigated to characterize the viscoelasticity of bladder cancer cells of various invasiveness on soft substrates, revealing that the rheology parameters are a signature of malignancy. Furthermore, the collagen microenvironment affects the viscoelastic moduli of cancer cell spheroids; thus, collagen serves as a powerful proxy, leading to an increase of the dynamic moduli vs. frequency, as predicted by a double power law model. Taken together, these results shed new light on how cancer cells and tissues adapt their viscoelastic properties depending on their malignancy and the microenvironment. This method could be an attractive way to control their properties in the future, based on the similarity of spheroids with in vivo tumor models.
Keywords: AFM; cancer cells; confocal microscopy; microenvironment; rheology; spheroids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Verdier C., Étienne J., Duperray A., Preziosi L. Review: Rheological properties of biological materials. C. R. Acad. Sci. Phys. 2009;10:790–811. doi: 10.1016/j.crhy.2009.10.003. - DOI
-
- Abidine Y., Laurent V.M., Michel R., Duperray A., Verdier C. Local mechanical properties of bladder cancer cells measured by AFM as a signature of metastatic potential. Eur. Phys. J. Plus. 2015;130:202. doi: 10.1140/epjp/i2015-15202-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

