The Vascular Circadian Clock in Chronic Kidney Disease
- PMID: 34359937
- PMCID: PMC8306728
- DOI: 10.3390/cells10071769
The Vascular Circadian Clock in Chronic Kidney Disease
Abstract
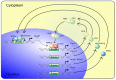
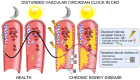
Chronic kidney disease is associated with extremely high cardiovascular mortality. The circadian rhythms (CR) have an impact on vascular function. The disruption of CR causes serious health problems and contributes to the development of cardiovascular diseases. Uremia may affect the master pacemaker of CR in the hypothalamus. A molecular circadian clock is also expressed in peripheral tissues, including the vasculature, where it regulates the different aspects of both vascular physiology and pathophysiology. Here, we address the impact of CKD on the intrinsic circadian clock in the vasculature. The expression of the core circadian clock genes in the aorta is disrupted in CKD. We propose a novel concept of the disruption of the circadian clock system in the vasculature of importance for the pathology of the uremic vasculopathy.
Keywords: ICAM-1; VCAM-1; adhesion molecules; cardiovascular disease; ccl2; inflammation; thrombomodulin; uremia; vascular calcification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

