Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction
- PMID: 34359963
- PMCID: PMC8304203
- DOI: 10.3390/cells10071794
Implant Fibrosis and the Underappreciated Role of Myofibroblasts in the Foreign Body Reaction
Abstract
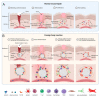
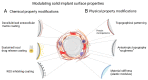
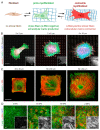
Body implants and implantable medical devices have dramatically improved and prolonged the life of countless patients. However, our body repair mechanisms have evolved to isolate, reject, or destroy any object that is recognized as foreign to the organism and inevitably mounts a foreign body reaction (FBR). Depending on its severity and chronicity, the FBR can impair implant performance or create severe clinical complications that will require surgical removal and/or replacement of the faulty device. The number of review articles discussing the FBR seems to be proportional to the number of different implant materials and clinical applications and one wonders, what else is there to tell? We will here take the position of a fibrosis researcher (which, coincidentally, we are) to elaborate similarities and differences between the FBR, normal wound healing, and chronic healing conditions that result in the development of peri-implant fibrosis. After giving credit to macrophages in the inflammatory phase of the FBR, we will mainly focus on the activation of fibroblastic cells into matrix-producing and highly contractile myofibroblasts. While fibrosis has been discussed to be a consequence of the disturbed and chronic inflammatory milieu in the FBR, direct activation of myofibroblasts at the implant surface is less commonly considered. Thus, we will provide a perspective how physical properties of the implant surface control myofibroblast actions and accumulation of stiff scar tissue. Because formation of scar tissue at the surface and around implant materials is a major reason for device failure and extraction surgeries, providing implant surfaces with myofibroblast-suppressing features is a first step to enhance implant acceptance and functional lifetime. Alternative therapeutic targets are elements of the myofibroblast mechanotransduction and contractile machinery and we will end with a brief overview on such targets that are considered for the treatment of other organ fibroses.
Keywords: TGF-β1; collagen; contracture; elastic modulus; fibroblast; macrophage; mechanosensing; micro-pattern; tissue repair; topography; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

