NF-κB-An Important Player in Xenoestrogen Signaling in Immune Cells
- PMID: 34359968
- PMCID: PMC8304139
- DOI: 10.3390/cells10071799
NF-κB-An Important Player in Xenoestrogen Signaling in Immune Cells
Abstract
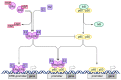
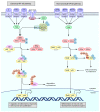
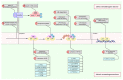
The proper functioning of the immune system is critical for an effective defense against pathogenic factors such as bacteria and viruses. All the cellular processes taking place in an organism are strictly regulated by an intracellular network of signaling pathways. In the case of immune cells, the NF-κB pathway is considered the key signaling pathway as it regulates the expression of more than 200 genes. The transcription factor NF-κB is sensitive to exogenous factors, such as xenoestrogens (XEs), which are compounds mimicking the action of endogenous estrogens and are widely distributed in the environment. Moreover, XE-induced modulation of signaling pathways may be crucial for the proper development of the immune system. In this review, we summarize the effects of XEs on the NF-κB signaling pathway. Based on our analysis, we constructed a model of XE-induced signaling in immune cells and found that in most cases XEs activate NF-κB. Our analysis indicated that the indirect impact of XEs on NF-κB in immune cells is related to the modulation of estrogen signaling and other pathways such as MAPK and JAK/STAT. We also summarize the role of these aspects of signaling in the development and further functioning of the immune system in this paper.
Keywords: NF-κB; endocrine disrupting chemicals; xenoestrogen signaling; xenoestrogens.
Conflict of interest statement
Authors declare no commercial or financial conflict of interest.
Figures

Similar articles
-
Serum amyloid A activates NF-kappaB and proinflammatory gene expression in human and murine intestinal epithelial cells.Eur J Immunol. 2005 Mar;35(3):718-26. doi: 10.1002/eji.200425688. Eur J Immunol. 2005. PMID: 15724247
-
Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes.J Bone Miner Res. 2006 Jan;21(1):37-47. doi: 10.1359/JBMR.050908. Epub 2005 Sep 19. J Bone Miner Res. 2006. PMID: 16355272
-
Methylparaben-induced regulation of estrogenic signaling in human neutrophils.Mol Cell Endocrinol. 2021 Dec 1;538:111470. doi: 10.1016/j.mce.2021.111470. Epub 2021 Oct 1. Mol Cell Endocrinol. 2021. PMID: 34606965
-
The role and impact of estrogens and xenoestrogen on the development of cervical cancer.Biomed Pharmacother. 2016 Dec;84:1945-1953. doi: 10.1016/j.biopha.2016.11.007. Epub 2016 Nov 15. Biomed Pharmacother. 2016. PMID: 27863841 Review.
-
Non-conventional signal transduction by type 1 interferons: the NF-kappaB pathway.J Cell Biochem. 2007 Dec 1;102(5):1087-94. doi: 10.1002/jcb.21535. J Cell Biochem. 2007. PMID: 17910035 Review.
Cited by
-
The roles and mechanisms of the NF-κB signaling pathway in tendon disorders.Front Vet Sci. 2024 Jun 24;11:1382239. doi: 10.3389/fvets.2024.1382239. eCollection 2024. Front Vet Sci. 2024. PMID: 38978635 Free PMC article. Review.
-
Identification of Shared Biomarkers in Chronic Kidney Disease and Diabetic Nephropathy Using Single-Cell Sequencing.Diabetes Metab Syndr Obes. 2025 Jul 5;18:2155-2174. doi: 10.2147/DMSO.S514319. eCollection 2025. Diabetes Metab Syndr Obes. 2025. PMID: 40636753 Free PMC article.
-
Omega-3 fatty acid ameliorates bisphenol F-induced testicular toxicity by modulating Nrf2/NFkB pathway and apoptotic signaling.Front Endocrinol (Lausanne). 2023 Sep 20;14:1256154. doi: 10.3389/fendo.2023.1256154. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37800144 Free PMC article.
-
Cracking the Code of Oocyte Quality: The Oxidative Stress Link to IVF Success.Int J Mol Sci. 2025 Jul 2;26(13):6377. doi: 10.3390/ijms26136377. Int J Mol Sci. 2025. PMID: 40650156 Free PMC article. Review.
-
Bisphenol A-What Do We Know? A Global or Local Approach at the Public Health Risk Level.Int J Mol Sci. 2024 Jun 5;25(11):6229. doi: 10.3390/ijms25116229. Int J Mol Sci. 2024. PMID: 38892416 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

