Trafficking to the Cell Surface of Amino Acid Transporter SLC6A14 Upregulated in Cancer Is Controlled by Phosphorylation of SEC24C Protein by AKT Kinase
- PMID: 34359969
- PMCID: PMC8307180
- DOI: 10.3390/cells10071800
Trafficking to the Cell Surface of Amino Acid Transporter SLC6A14 Upregulated in Cancer Is Controlled by Phosphorylation of SEC24C Protein by AKT Kinase
Abstract
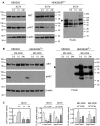
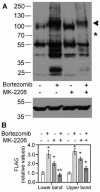
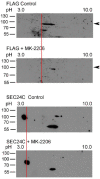
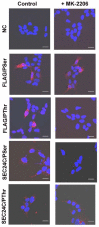
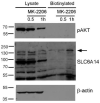
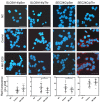
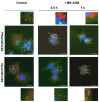
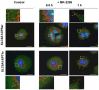
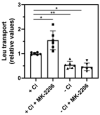
Cancer cells need a constant supply of nutrients. SLC6A14, an amino acid transporter B0,+ (ATB0,+) that is upregulated in many cancers, transports all but acidic amino acids. In its exit from the endoplasmic reticulum (ER), it is recognized by the SEC24C subunit of coatomer II (COPII) for further vesicular trafficking to the plasma membrane. SEC24C has previously been shown to be phosphorylated by protein kinase B/AKT, which is hyper-activated in cancer; therefore, we analyzed the influence of AKT on SLC6A14 trafficking to the cell surface. Studies on overexpressed and endogenous transporters in the breast cancer cell line MCF-7 showed that AKT inhibition with MK-2206 correlated with a transient increase of the transporter in the plasma membrane, not resulting from the inhibition of ER-associated protein degradation. Two-dimensional electrophoresis demonstrated the decreased phosphorylation of SLC6A14 and SEC24C upon AKT inhibition. A proximity ligation assay confirmed this conclusion: AKT inhibition is correlated with decreased SLC6A14 phosphothreonine and SEC24C phosphoserine. Augmented levels of SLC6A14 in plasma membrane led to increased leucine transport. These results show that the inactivation of AKT can rescue amino acid delivery through SLC6A14 trafficking to the cell surface, supporting cancer cell survival. The regulation of the ER export of the amino acid transporter seems to be a novel function of AKT.
Keywords: AKT kinase; SEC24C; SLC6A14; amino acid transporter; breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Trafficking of the amino acid transporter B0,+ (SLC6A14) to the plasma membrane involves an exclusive interaction with SEC24C for its exit from the endoplasmic reticulum.Biochim Biophys Acta Mol Cell Res. 2019 Feb;1866(2):252-263. doi: 10.1016/j.bbamcr.2018.11.005. Epub 2018 Nov 14. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30445147 Free PMC article.
-
Amino Acid Transporter SLC6A14 (ATB0,+) - A Target in Combined Anti-cancer Therapy.Front Cell Dev Biol. 2020 Oct 21;8:594464. doi: 10.3389/fcell.2020.594464. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195271 Free PMC article. Review.
-
Amino acid transporter SLC6A14 depends on heat shock protein HSP90 in trafficking to the cell surface.Biochim Biophys Acta Mol Cell Res. 2019 Oct;1866(10):1544-1555. doi: 10.1016/j.bbamcr.2019.07.009. Epub 2019 Jul 18. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31326539
-
Regulation of SLC6A14 trafficking in breast cancer cells by heat shock protein HSP90β.Biochem Biophys Res Commun. 2022 Jul 23;614:41-46. doi: 10.1016/j.bbrc.2022.05.011. Epub 2022 May 6. Biochem Biophys Res Commun. 2022. PMID: 35569376
-
Vesicle-mediated export from the ER: COPII coat function and regulation.Biochim Biophys Acta. 2013 Nov;1833(11):2464-72. doi: 10.1016/j.bbamcr.2013.02.003. Epub 2013 Feb 15. Biochim Biophys Acta. 2013. PMID: 23419775 Free PMC article. Review.
Cited by
-
Transmembrane Amino Acid Transporters in Shaping the Metabolic Profile of Breast Cancer Cell Lines: The Focus on Molecular Biological Subtype.Curr Issues Mol Biol. 2024 Dec 25;47(1):4. doi: 10.3390/cimb47010004. Curr Issues Mol Biol. 2024. PMID: 39852119 Free PMC article. Review.
-
Screening of ulcerative colitis biomarkers and potential pathways based on weighted gene co-expression network, machine learning and ceRNA hypothesis.Hereditas. 2022 Nov 23;159(1):42. doi: 10.1186/s41065-022-00259-4. Hereditas. 2022. PMID: 36419192 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

