LncRNA MORT (ZNF667-AS1) in Cancer-Is There a Possible Role in Gynecological Malignancies?
- PMID: 34360598
- PMCID: PMC8346052
- DOI: 10.3390/ijms22157829
LncRNA MORT (ZNF667-AS1) in Cancer-Is There a Possible Role in Gynecological Malignancies?
Abstract
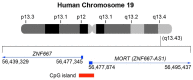
Gynecological cancers (GCs) are currently among the major threats to female health. Moreover, there are different histologic subtypes of these cancers, which are defined as 'rare' due to an annual incidence of <6 per 100,000 women. The majority of these tend to be associated with a poor prognosis. Long non-coding RNAs (lncRNAs) play a critical role in the normal development of organisms as well as in tumorigenesis. LncRNAs can be classified into tumor suppressor genes or oncogenes, depending on their function within the cellular context and the signaling pathways in which they are involved. These regulatory RNAs are potential therapeutic targets for cancer due to their tissue and tumor specificity. However, there still needs to be a deeper understanding of the mechanisms by which lncRNAs are involved in the regulation of numerous biological functions in humans, both in normal health and disease. The lncRNA Mortal Obligate RNA Transcript (MORT; alias ZNF667-AS1) has been identified as a tumor-related lncRNA. ZNF667-AS1 gene, located in the human chromosome region 19q13.43, has been shown to be silenced by DNA hypermethylation in several cancers. In this review, we report on the biological functions of ZNF667-AS1 from recent studies and describe the regulatory functions of ZNF667-AS1 in human disease, including cancer. Furthermore, we discuss the emerging insights into the potential role of ZNF667-AS1 as a biomarker and novel therapeutic target in cancer, including GCs (ovarian, cervical, and endometrial cancers).
Keywords: MORT (ZNF667-AS1); cervical cancer; endometrial cancer; epigenetic therapy; epigenetics; lncRNA-based therapy; long non-coding RNA; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Mandilaras V., Karakasis K., Clarke B., Amit Oza A., Lheureux S. Rare tumors in gynaecological cancers and the lack of therapeutic options and clinical trials. Expert Opin. Orphan Drugs. 2017;5:71–83. doi: 10.1080/21678707.2017.1264300. - DOI
-
- Ray-Coquard I., Trama A., Seckl M.J., Fotopoulou C., Pautier P., Pignata S., Kristensen G., Mangili G., Falconer H., Massuger L., et al. Rare ovarian tumours: Epidemiology, treatment challenges in and outside a network setting. Eur. J. Surg. Oncol. 2017;45:67–74. doi: 10.1016/j.ejso.2017.09.025. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

