Cytokine-Laden Extracellular Vesicles Predict Patient Prognosis after Cerebrovascular Accident
- PMID: 34360613
- PMCID: PMC8345931
- DOI: 10.3390/ijms22157847
Cytokine-Laden Extracellular Vesicles Predict Patient Prognosis after Cerebrovascular Accident
Abstract
Background: A major contributor to disability after hemorrhagic stroke is secondary brain damage induced by the inflammatory response. Following stroke, global increases in numerous cytokines-many associated with worse outcomes-occur within the brain, cerebrospinal fluid, and peripheral blood. Extracellular vesicles (EVs) may traffic inflammatory cytokines from damaged tissue within the brain, as well as peripheral sources, across the blood-brain barrier, and they may be a critical component of post-stroke neuroinflammatory signaling.
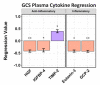
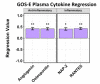
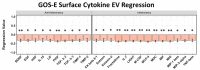
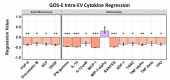
Methods: We performed a comprehensive analysis of cytokine concentrations bound to plasma EV surfaces and/or sequestered within the vesicles themselves. These concentrations were correlated to patient acute neurological condition by the Glasgow Coma Scale (GCS) and to chronic, long-term outcome via the Glasgow Outcome Scale-Extended (GOS-E).
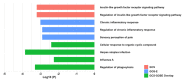
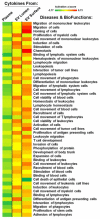
Results: Pro-inflammatory cytokines detected from plasma EVs were correlated to worse outcomes in hemorrhagic stroke patients. Anti-inflammatory cytokines detected within EVs were still correlated to poor outcomes despite their putative neuroprotective properties. Inflammatory cytokines macrophage-derived chemokine (MDC/CCL2), colony stimulating factor 1 (CSF1), interleukin 7 (IL7), and monokine induced by gamma interferon (MIG/CXCL9) were significantly correlated to both negative GCS and GOS-E when bound to plasma EV membranes.
Conclusions: These findings correlate plasma-derived EV cytokine content with detrimental outcomes after stroke, highlighting the potential for EVs to provide cytokines with a means of long-range delivery of inflammatory signals that perpetuate neuroinflammation after stroke, thus hindering recovery.
Keywords: Glasgow Coma Scale-Extended; Glasgow Outcome Scale; chemotaxis; cytokines; extracellular vesicles; hemorrhagic stroke; inflammation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., de Ferranti S.D., Floyd J., Fornage M., Gillespie C., et al. Heart disease and stroke statistics-2017 update: A report from the american heart association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. - DOI - PMC - PubMed
-
- Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

