Mucus Release and Airway Constriction by TMEM16A May Worsen Pathology in Inflammatory Lung Disease
- PMID: 34360618
- PMCID: PMC8346050
- DOI: 10.3390/ijms22157852
Mucus Release and Airway Constriction by TMEM16A May Worsen Pathology in Inflammatory Lung Disease
Abstract
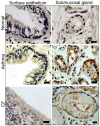
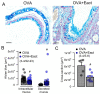
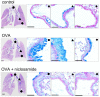
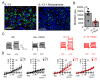
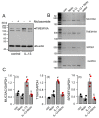
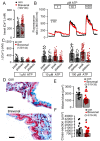
Activation of the Ca2+ activated Cl- channel TMEM16A is proposed as a treatment in inflammatory airway disease. It is assumed that activation of TMEM16A will induce electrolyte secretion, and thus reduce airway mucus plugging and improve mucociliary clearance. A benefit of activation of TMEM16A was shown in vitro and in studies in sheep, but others reported an increase in mucus production and airway contraction by activation of TMEM16A. We analyzed expression of TMEM16A in healthy and inflamed human and mouse airways and examined the consequences of activation or inhibition of TMEM16A in asthmatic mice. TMEM16A was found to be upregulated in the lungs of patients with asthma or cystic fibrosis, as well as in the airways of asthmatic mice. Activation or potentiation of TMEM16A by the compounds Eact or brevenal, respectively, induced acute mucus release from airway goblet cells and induced bronchoconstriction in mice in vivo. In contrast, niclosamide, an inhibitor of TMEM16A, blocked mucus production and mucus secretion in vivo and in vitro. Treatment of airway epithelial cells with niclosamide strongly inhibited expression of the essential transcription factor of Th2-dependent inflammation and goblet cell differentiation, SAM pointed domain-containing ETS-like factor (SPDEF). Activation of TMEM16A in people with inflammatory airway diseases is likely to induce mucus secretion along with airway constriction. In contrast, inhibitors of TMEM16A may suppress pulmonary Th2 inflammation, goblet cell metaplasia, mucus production, and bronchoconstriction, partially by inhibiting expression of SPDEF.
Keywords: ETX001; Eact; TMEM16A; airways; asthma; brevenal; cystic fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mall M., Gonska T., Thomas J., Schreiber R., Seydewitz H.H., Kuehr J., Brandis M., Kunzelmann K. Modulation of Ca2+ activated Cl− secretion by basolateral K+ channels in human normal and cystic fibrosis airway epithelia. Pediatr. Res. 2003;53:608–618. doi: 10.1203/01.PDR.0000057204.51420.DC. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

