Tumor Promoting Effect of BMP Signaling in Endometrial Cancer
- PMID: 34360647
- PMCID: PMC8346149
- DOI: 10.3390/ijms22157882
Tumor Promoting Effect of BMP Signaling in Endometrial Cancer
Abstract
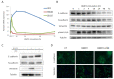
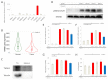
The effects of bone morphogenetic proteins (BMPs), members of the transforming growth factor-β (TGF-β) family, in endometrial cancer (EC) have yet to be determined. In this study, we analyzed the TCGA and MSK-IMPACT datasets and investigated the effects of BMP2 and of TWSG1, a BMP antagonist, on Ishikawa EC cells. Frequent ACVR1 mutations and high mRNA expressions of BMP ligands and receptors were observed in EC patients of the TCGA and MSK-IMPACT datasets. Ishikawa cells secreted higher amounts of BMP2 compared with ovarian cancer cell lines. Exogenous BMP2 stimulation enhanced EC cell sphere formation via c-KIT induction. BMP2 also induced EMT of EC cells, and promoted migration by induction of SLUG. The BMP receptor kinase inhibitor LDN193189 augmented the growth inhibitory effects of carboplatin. Analyses of mRNAs of several BMP antagonists revealed that TWSG1 mRNA was abundantly expressed in Ishikawa cells. TWSG1 suppressed BMP7-induced, but not BMP2-induced, EC cell sphere formation and migration. Our results suggest that BMP signaling promotes EC tumorigenesis, and that TWSG1 antagonizes BMP7 in EC. BMP signaling inhibitors, in combination with chemotherapy, might be useful in the treatment of EC patients.
Keywords: ACVR1; BMP; EMT; cancer stem cells; endometrial cancer.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

