Chirality Matters: Biological Activity of Optically Pure Silybin and Its Congeners
- PMID: 34360650
- PMCID: PMC8346157
- DOI: 10.3390/ijms22157885
Chirality Matters: Biological Activity of Optically Pure Silybin and Its Congeners
Abstract
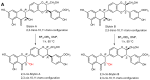
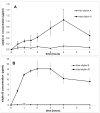
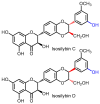
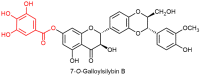
This review focuses on the specific biological effects of optically pure silymarin flavo-nolignans, mainly silybins A and B, isosilybins A and B, silychristins A and B, and their 2,3-dehydro derivatives. The chirality of these flavonolignans is also discussed in terms of their analysis, preparative separation and chemical reactions. We demonstrated the specific activities of the respective diastereomers of flavonolignans and also the enantiomers of their 2,3-dehydro derivatives in the 3D anisotropic systems typically represented by biological systems. In vivo, silymarin flavonolignans do not act as redox antioxidants, but they play a role as specific ligands of biological targets, according to the "lock-and-key" concept. Estrogenic, antidiabetic, anticancer, antiviral, and antiparasitic effects have been demonstrated in optically pure flavonolignans. Potential application of pure flavonolignans has also been shown in cardiovascular and neurological diseases. Inhibition of drug-metabolizing enzymes and modulation of multidrug resistance activity by these compounds are discussed in detail. The future of "silymarin applications" lies in the use of optically pure components that can be applied directly or used as valuable lead structures, and in the exploration of their true molecular effects.
Keywords: Silybum marianum; chirality; dehydroflavonolignan; diastereomer; flavonoid; flavonolignan; isosilybin; milk thistle; silibinin; silybin; silychristin; silydianin; silymarin.
Conflict of interest statement
The author declares no conflict of interest.
Figures









References
-
- Egea J., Fabregat I., Frapart Y.M., Ghezzi P., Görlach A., Kietzmann T., Kubaichuk K., Knaus U.G., Lopez M.G., Olaso-Gonzalez G., et al. European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS) Redox Biol. 2017;13:94–162. doi: 10.1016/j.redox.2017.05.007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

