The Evolving Roles of Cardiac Macrophages in Homeostasis, Regeneration, and Repair
- PMID: 34360689
- PMCID: PMC8347787
- DOI: 10.3390/ijms22157923
The Evolving Roles of Cardiac Macrophages in Homeostasis, Regeneration, and Repair
Abstract
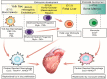
Macrophages were first described as phagocytic immune cells responsible for maintaining tissue homeostasis by the removal of pathogens that disturb normal function. Historically, macrophages have been viewed as terminally differentiated monocyte-derived cells that originated through hematopoiesis and infiltrated multiple tissues in the presence of inflammation or during turnover in normal homeostasis. However, improved cell detection and fate-mapping strategies have elucidated the various lineages of tissue-resident macrophages, which can derive from embryonic origins independent of hematopoiesis and monocyte infiltration. The role of resident macrophages in organs such as the skin, liver, and the lungs have been well characterized, revealing functions well beyond a pure phagocytic and immunological role. In the heart, recent research has begun to decipher the functional roles of various tissue-resident macrophage populations through fate mapping and genetic depletion studies. Several of these studies have elucidated the novel and unexpected roles of cardiac-resident macrophages in homeostasis, including maintaining mitochondrial function, facilitating cardiac conduction, coronary development, and lymphangiogenesis, among others. Additionally, following cardiac injury, cardiac-resident macrophages adopt diverse functions such as the clearance of necrotic and apoptotic cells and debris, a reduction in the inflammatory monocyte infiltration, promotion of angiogenesis, amelioration of inflammation, and hypertrophy in the remaining myocardium, overall limiting damage extension. The present review discusses the origin, development, characterization, and function of cardiac macrophages in homeostasis, cardiac regeneration, and after cardiac injury or stress.
Keywords: cardiac homeostasis; inflammation; macrophages; monocytes; myocardial infarction; regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hoeffel G., Wang Y., Greter M., See P., Teo P., Malleret B., Leboeuf M., Low D., Oller G., Almeida F., et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

