Osteoblast Dysfunction in Non-Hereditary Sclerosing Bone Diseases
- PMID: 34360745
- PMCID: PMC8348499
- DOI: 10.3390/ijms22157980
Osteoblast Dysfunction in Non-Hereditary Sclerosing Bone Diseases
Abstract
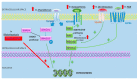
A review of the available literature was performed in order to summarize the existing evidence between osteoblast dysfunction and clinical features in non-hereditary sclerosing bone diseases. It has been known that proliferation and migration of osteoblasts are concerted by soluble factors such as fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor (TGF), bone morphogenetic protein (BMP) but also by signal transduction cascades such as Wnt signaling pathway. Protein kinases play also a leading role in triggering the activation of osteoblasts in this group of diseases. Post-zygotic changes in mitogen-activated protein kinase (MAPK) have been shown to be associated with sporadic cases of Melorheostosis. Serum levels of FGF and PDGF have been shown to be increased in myelofibrosis, although studies focusing on Sphingosine-1-phosphate receptor was shown to be strongly expressed in Paget disease of the bone, which may partially explain the osteoblastic hyperactivity during this condition. Pathophysiological mechanisms of osteoblasts in osteoblastic metastases have been studied much more thoroughly than in rare sclerosing syndromes: striking cellular mechanisms such as osteomimicry or complex intercellular signaling alterations have been described. Further research is needed to describe pathological mechanisms by which rare sclerosing non hereditary diseases lead to osteoblast dysfunction.
Keywords: bone metabolism; bone sclerosis; melorheostosis; osteoblasts; osteocondensation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hayashi M., Ono T., Nakashima T. Signaling in Osteoblast Differentiation, Zaidi MBT-E. of BB, ed. Academic Press; Oxford, UK: 2020. pp. 416–426. - DOI
-
- Sugamori Y., Mise-Omata S., Maeda C., Aoki S., Tabata Y., Murali R., Yasuda H., Udagawa N., Suzuki H., Honma M., et al. Peptide drugs accelerate BMP-2-induced calvarial bone regeneration and stimulate osteoblast differentiation through mTORC1 signaling. BioEssays News Rev. Mol. Cell. Dev. Biol. 2016;38:717–725. doi: 10.1002/bies.201600104. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources