Metal Oxide Nanoparticles: Evidence of Adverse Effects on the Male Reproductive System
- PMID: 34360825
- PMCID: PMC8348343
- DOI: 10.3390/ijms22158061
Metal Oxide Nanoparticles: Evidence of Adverse Effects on the Male Reproductive System
Abstract
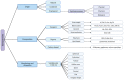
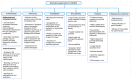
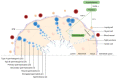
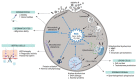
Metal oxide nanoparticles (MONPs) are inorganic materials that have become a valuable tool for many industrial sectors, especially in healthcare, due to their versatility, unique intrinsic properties, and relatively inexpensive production cost. As a consequence of their wide applications, human exposure to MONPs has increased dramatically. More recently, their use has become somehow controversial. On one hand, MONPs can interact with cellular macromolecules, which makes them useful platforms for diagnostic and therapeutic interventions. On the other hand, research suggests that these MONPs can cross the blood-testis barrier and accumulate in the testis. Although it has been demonstrated that some MONPs have protective effects on male germ cells, contradictory reports suggest that these nanoparticles compromise male fertility by interfering with spermatogenesis. In fact, in vitro and in vivo studies indicate that exposure to MONPs could induce the overproduction of reactive oxygen species, resulting in oxidative stress, which is the main suggested molecular mechanism that leads to germ cells' toxicity. The latter results in subsequent damage to proteins, cell membranes, and DNA, which ultimately may lead to the impairment of the male reproductive system. The present manuscript overviews the therapeutic potential of MONPs and their biomedical applications, followed by a critical view of their potential risks in mammalian male fertility, as suggested by recent scientific literature.
Keywords: biomedicine; male infertility; metal-oxide nanoparticles; nanotoxicity; oxidative stress; reproductive system; spermatogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lövestam G., Rauscher H., Roebben G., Klüttgen B., Gibson N., Putaud J.-P., Stamm H. Considerations on a Definition of Nanomaterial for Regulatory Purposes. Publications Office of the European Union; Luxembourg: 2010. JRC Reference Reports.
-
- Khan I., Saeed K., Khan I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019;12:908–931. doi: 10.1016/j.arabjc.2017.05.011. - DOI
-
- Singla R., Guliani A., Kumari A., Yadav S.K. Metallic Nanoparticles, Toxicity Issues and Applications in Medicine. Springer; Singapore: 2016.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

