Inflammation, Extracellular Matrix Remodeling, and Proteostasis in Tumor Microenvironment
- PMID: 34360868
- PMCID: PMC8346982
- DOI: 10.3390/ijms22158102
Inflammation, Extracellular Matrix Remodeling, and Proteostasis in Tumor Microenvironment
Abstract
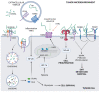
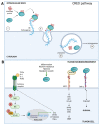
Cancer is a multifaceted and complex pathology characterized by uncontrolled cell proliferation and decreased apoptosis. Most cancers are recognized by an inflammatory environment rich in a myriad of factors produced by immune infiltrate cells that induce host cells to differentiate and to produce a matrix that is more favorable to tumor cells' survival and metastasis. As a result, the extracellular matrix (ECM) is changed in terms of macromolecules content, degrading enzymes, and proteins. Altered ECM components, derived from remodeling processes, interact with a variety of surface receptors triggering intracellular signaling that, in turn, cancer cells exploit to their own benefit. This review aims to present the role of different aspects of ECM components in the tumor microenvironment. Particularly, we highlight the effect of pro- and inflammatory factors on ECM degrading enzymes, such as metalloproteases, and in a more detailed manner on hyaluronan metabolism and the signaling pathways triggered by the binding of hyaluronan with its receptors. In addition, we sought to explore the role of extracellular chaperones, especially of clusterin which is one of the most prominent in the extracellular space, in proteostasis and signaling transduction in the tumor microenvironment. Although the described tumor microenvironment components have different biological roles, they may engage common signaling pathways that favor tumor growth and metastasis.
Keywords: clusterin; extracellular chaperones; extracellular matrix remodeling; hyaluronan; inflammation; matrix metalloproteases; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

