Staphylococcus aureus Decreases SUMOylation Host Response to Promote Intramacrophage Survival
- PMID: 34360873
- PMCID: PMC8347835
- DOI: 10.3390/ijms22158108
Staphylococcus aureus Decreases SUMOylation Host Response to Promote Intramacrophage Survival
Abstract
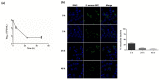
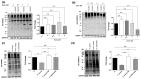
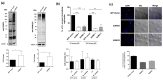
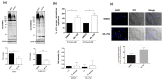
Staphylococcus aureus is a commensal bacterium that causes severe infections in soft tissue and the bloodstream. During infection, S. aureus manipulates host cell response to facilitate its own replication and dissemination. Here, we show that S. aureus significantly decreases the level of SUMOylation, an essential post-translational modification, in infected macrophages 24 h post-phagocytosis. The reduced level of SUMOylation correlates with a decrease in the SUMO-conjugating enzyme Ubc9. The over-expression of SUMO proteins in macrophages impaired bacterial intracellular proliferation and the inhibition of SUMOylation with ML-792 increased it. Together, these findings demonstrated for the first time the role of host SUMOylation response toward S. aureus infection.
Keywords: SUMOylation; Staphylococcus aureus; Ubc9; infection; intracellular survival; macrophage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kubica M., Guzik K., Koziel J., Zarebski M., Richter W., Gajkowska B., Golda A., Maciag-Gudowska A., Brix K., Shaw L., et al. A Potential New Pathway for Staphylococcus aureus Dissemination: The Silent Survival of S. aureus Phagocytosed by Human Monocyte-Derived Macrophages. PLoS ONE. 2008;3:e1409. doi: 10.1371/journal.pone.0001409. - DOI - PMC - PubMed
-
- Lacoma A., Cano V., Moranta D., Regueiro V., Domínguez-Villanueva D., Laabei M., González-Nicolau M., Ausina V., Prat C., Bengoechea J.A. Investigating Intracellular Persistence of Staphylococcus aureus within a Murine Alveolar Macrophage Cell Line. Virulence. 2017;8:1761–1775. doi: 10.1080/21505594.2017.1361089. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

