An Evaluation of Human Induced Pluripotent Stem Cells to Test for Cardiac Developmental Toxicity
- PMID: 34360880
- PMCID: PMC8347148
- DOI: 10.3390/ijms22158114
An Evaluation of Human Induced Pluripotent Stem Cells to Test for Cardiac Developmental Toxicity
Abstract
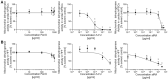
To prevent congenital defects arising from maternal exposure, safety regulations require pre-market developmental toxicity screens for industrial chemicals and pharmaceuticals. Traditional embryotoxicity approaches depend heavily on the use of low-throughput animal models which may not adequately predict human risk. The validated embryonic stem cell test (EST) developed in murine embryonic stem cells addressed the former problem over 15 years ago. Here, we present a proof-of-concept study to address the latter challenge by updating all three endpoints of the classic mouse EST with endpoints derived from human induced pluripotent stem cells (hiPSCs) and human fibroblasts. Exposure of hiPSCs to selected test chemicals inhibited differentiation at lower concentrations than observed in the mouse EST. The hiPSC-EST also discerned adverse developmental outcomes driven by novel environmental toxicants. Evaluation of the early cardiac gene TBX5 yielded similar toxicity patterns as the full-length hiPSC-EST. Together, these findings support the further development of hiPSCs and early molecular endpoints as a biologically relevant embryotoxicity screening approach for individual chemicals and mixtures.
Keywords: cardiomyocytes; embryonic stem cell test; embryotoxicity screen; induced pluripotent stem cells; tobacco smoke solution.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER) Guidance for Industry Reproductive and Developmental Toxicities—Integrating Study Results to Assess Concerns. Food and Drug Administration Center for Drug Evaluation and Research (CDER); Silver Spring, MD, USA: 2011.
-
- Center for Food Safety and Applied Nutrition . Redbook 2000: IV.C.9.b Guidelines for Developmental Toxicity Studies. Center for Food Safety and Applied Nutrition; College Park, MD, USA: 2000.
-
- Augustine-Rauch K., Zhang C.X., Panzica-Kelly J.M. In vitro developmental toxicology assays: A review of the state of the science of rodent and zebrafish whole embryo culture and embryonic stem cell assays. Birth Defects Res. Part C Embryo Today Rev. 2010;90:87–98. doi: 10.1002/bdrc.20175. - DOI - PubMed
-
- Spielmann H., Pohl I., Doring B., Liebsch M., Moldenhauer F. The embryonic stem cell test (EST), an in vitro embryotoxicity test using two permanent mouse cell lines: 3T3 fibroblasts and embryonic stem cells. In Vitro Toxicol. 1997;10:119–127.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

