Mitochondrial Modulations, Autophagy Pathways Shifts in Viral Infections: Consequences of COVID-19
- PMID: 34360945
- PMCID: PMC8347486
- DOI: 10.3390/ijms22158180
Mitochondrial Modulations, Autophagy Pathways Shifts in Viral Infections: Consequences of COVID-19
Abstract
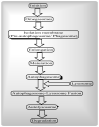
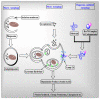
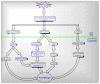
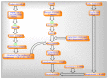
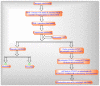
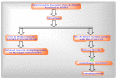
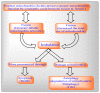
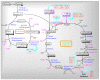
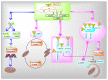
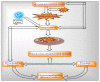
Mitochondria are vital intracellular organelles that play an important role in regulating various intracellular events such as metabolism, bioenergetics, cell death (apoptosis), and innate immune signaling. Mitochondrial fission, fusion, and membrane potential play a central role in maintaining mitochondrial dynamics and the overall shape of mitochondria. Viruses change the dynamics of the mitochondria by altering the mitochondrial processes/functions, such as autophagy, mitophagy, and enzymes involved in metabolism. In addition, viruses decrease the supply of energy to the mitochondria in the form of ATP, causing viruses to create cellular stress by generating ROS in mitochondria to instigate viral proliferation, a process which causes both intra- and extra-mitochondrial damage. SARS-COV2 propagates through altering or changing various pathways, such as autophagy, UPR stress, MPTP and NLRP3 inflammasome. Thus, these pathways act as potential targets for viruses to facilitate their proliferation. Autophagy plays an essential role in SARS-COV2-mediated COVID-19 and modulates autophagy by using various drugs that act on potential targets of the virus to inhibit and treat viral infection. Modulated autophagy inhibits coronavirus replication; thus, it becomes a promising target for anti-coronaviral therapy. This review gives immense knowledge about the infections, mitochondrial modulations, and therapeutic targets of viruses.
Keywords: COVID-19; SARS-COV2; autophagy; mitochondria; potential targets; viral infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

