A Short-Type Peptidoglycan Recognition Protein 1 (PGRP1) Is Involved in the Immune Response in Asian Corn Borer, Ostrinia furnacalis (Guenée)
- PMID: 34360963
- PMCID: PMC8347126
- DOI: 10.3390/ijms22158198
A Short-Type Peptidoglycan Recognition Protein 1 (PGRP1) Is Involved in the Immune Response in Asian Corn Borer, Ostrinia furnacalis (Guenée)
Abstract
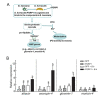
The insect immune response is initiated by the recognition of invading microorganisms. Peptidoglycan recognition proteins (PGRPs) function primarily as pattern recognition receptors by specifically binding to peptidoglycans expressed on microbial surfaces. We cloned a full-length cDNA for a PGRP from the Asian corn borer Ostrinia furnacalis (Guenée) and designated it as PGRP1. PGRP1 mRNA was mainly detected in the fat bodies and hemocytes. Its transcript levels increased significantly upon bacterial and fungal challenges. Purified recombinant PGRP1 exhibited binding activity to the gram-positive Micrococcus luteus, gram-negative Escherichia coli, entomopathogenic fungi Beauveria bassiana, and yeast Pichia pastoris. The binding further induced their agglutination. Additionally, PGRP1 preferred to bind to Lys-type peptidoglycans rather than DAP-type peptidoglycans. The addition of recombinant PGRP1 to O. furnacalis plasma resulted in a significant increase in phenoloxidase activity. The injection of recombinant PGRP1 into larvae led to a significantly increased expression of several antimicrobial peptide genes. Taken together, our results suggest that O. furnacalis PGRP1 potentially recognizes the invading microbes and is involved in the immune response in O. furnacalis.
Keywords: Ostrinia furnacalis (Guenée); agglutination; binding; innate immune response; peptidoglycan recognition proteins (PGRPs); prophenoloxidase stimulation.
Conflict of interest statement
All authors have seen and agree with the contents of the manuscript, and there are no conflicts of interest, including specific financial interests, relationships, and affiliations relevant to the subject of the manuscript.
Figures






References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

