Tunneling Quantum Dynamics in Ammonia
- PMID: 34361046
- PMCID: PMC8348077
- DOI: 10.3390/ijms22158282
Tunneling Quantum Dynamics in Ammonia
Abstract
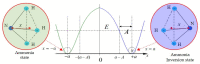
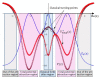
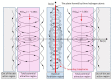
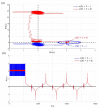
Ammonia is a well-known example of a two-state system and must be described in quantum-mechanical terms. In this article, we will explain the tunneling phenomenon that occurs in ammonia molecules from the perspective of trajectory-based quantum dynamics, rather than the usual quantum probability perspective. The tunneling of the nitrogen atom through the potential barrier in ammonia is not merely a probability problem; there are underlying reasons and mechanisms explaining why and how the tunneling in ammonia can happen. Under the framework of quantum Hamilton mechanics, the tunneling motion of the nitrogen atom in ammonia can be described deterministically in terms of the quantum trajectories of the nitrogen atom and the quantum forces applied. The vibrations of the nitrogen atom about its two equilibrium positions are analyzed in terms of its quantum trajectories, which are solved from the Hamilton equations of motion. The vibration periods are then computed by the quantum trajectories and compared with the experimental measurements.
Keywords: ammonia; quantum Hamilton mechanics; quantum molecular dynamics; quantum trajectory; tunneling dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Zhong S.Q., Zhao S.C., Zhu S.N. Photovoltaic properties enhanced by the tunneling effect in a coupled quantum dot photocell. Res. Phys. 2021;4:104094.
-
- Saldaña J.C.E., Vekris A., Žitko R., Steffensen G., Krogstrup P., Paaske J., Grove-Rasmussen K., Nygård J. Two-impurity Yu-Shiba-Rusinov states in coupled quantum dots. Phys. Rev. B. 2020;102:195143. doi: 10.1103/PhysRevB.102.195143. - DOI
-
- Li Q., Chen S., Yu H., Chen J., Yan X., Li L., Xu M.-W. Overcoming the conductivity limit of insulator through tunneling-current junction welding: Ag @ PVP core–shell nanowire for high-performance transparent electrode. J. Mater. Chem. C. 2021;9:3957–3968. doi: 10.1039/D0TC05783E. - DOI
-
- Huang H., Padurariu C., Ast C.R. Tunneling dynamics between superconducting bound states at the atomic limit. Nat. Phys. 2020;16:1227–1231. doi: 10.1038/s41567-020-0971-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

