Signaling Pathways Regulated by UBR Box-Containing E3 Ligases
- PMID: 34361089
- PMCID: PMC8346999
- DOI: 10.3390/ijms22158323
Signaling Pathways Regulated by UBR Box-Containing E3 Ligases
Abstract
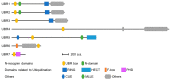
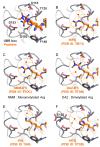
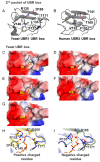
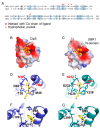
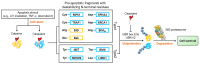
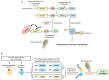
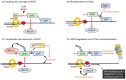
UBR box E3 ligases, also called N-recognins, are integral components of the N-degron pathway. Representative N-recognins include UBR1, UBR2, UBR4, and UBR5, and they bind destabilizing N-terminal residues, termed N-degrons. Understanding the molecular bases of their substrate recognition and the biological impact of the clearance of their substrates on cellular signaling pathways can provide valuable insights into the regulation of these pathways. This review provides an overview of the current knowledge of the binding mechanism of UBR box N-recognin/N-degron interactions and their roles in signaling pathways linked to G-protein-coupled receptors, apoptosis, mitochondrial quality control, inflammation, and DNA damage. The targeting of these UBR box N-recognins can provide potential therapies to treat diseases such as cancer and neurodegenerative diseases.
Keywords: Arg/N-degron pathway; DNA damage; G-protein signaling; N-degron; N-recognin; UBR Box E3 ligases; apoptosis; inflammatory response; mitochondrial quality control.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- King B., Trimarchi T., Reavie L., Xu L., Mullenders J., Ntziachristos P., Aranda-Orgilles B., Perez-Garcia A., Shi J., Vakoc C., et al. The ubiquitin ligase FBXW7 modulates leukemia-initiating cell activity by regulating MYC stability. Cell. 2013;153:1552–1566. doi: 10.1016/j.cell.2013.05.041. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

