Inflammasome Signaling Regulates the Microbial-Neuroimmune Axis and Visceral Pain in Mice
- PMID: 34361102
- PMCID: PMC8371481
- DOI: 10.3390/ijms22158336
Inflammasome Signaling Regulates the Microbial-Neuroimmune Axis and Visceral Pain in Mice
Abstract
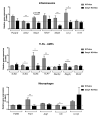
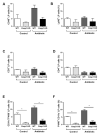
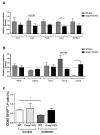
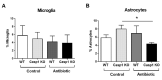
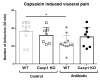
Interactions between the intestinal microbiota, immune system and nervous system are essential for homeostasis in the gut. Inflammasomes contribute to innate immunity and brain-gut interactions, but their role in microbiota-neuro-immune interactions is not clear. Therefore, we investigated the effect of the inflammasome on visceral pain and local and systemic neuroimmune responses after antibiotic-induced changes to the microbiota. Wild-type (WT) and caspase-1/11 deficient (Casp1 KO) mice were orally treated for 2 weeks with an antibiotic cocktail (Abx, Bacitracin A and Neomycin), followed by quantification of representative fecal commensals (by qPCR), cecal short chain fatty acids (by HPLC), pathways implicated in the gut-neuro-immune axis (by RT-qPCR, immunofluorescence staining, and flow cytometry) in addition to capsaicin-induced visceral pain responses. Abx-treatment in WT-mice resulted in an increase in colonic macrophages, central neuro-immune interactions, colonic inflammasome and nociceptive receptor gene expression and a reduction in capsaicin-induced visceral pain. In contrast, these responses were attenuated in Abx-treated Casp1 KO mice. Collectively, the data indicate an important role for the inflammasome pathway in functional and inflammatory gastrointestinal conditions where pain and alterations in microbiota composition are prominent.
Keywords: gut commensal microbiota; gut–brain axis; immune system; inflammasome.
Conflict of interest statement
FS is the co-founder/shareholder of Alimentary Health Ltd., Tucana Health Ltd. and Atlantia Food Clinical Trials Ltd. and scientific advisor to Kaleido Biosciences. All other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

