Advanced Optogenetic-Based Biosensing and Related Biomaterials
- PMID: 34361345
- PMCID: PMC8347019
- DOI: 10.3390/ma14154151
Advanced Optogenetic-Based Biosensing and Related Biomaterials
Abstract
The ability to stimulate mammalian cells with light, brought along by optogenetic control, has significantly broadened our understanding of electrically excitable tissues. Backed by advanced (bio)materials, it has recently paved the way towards novel biosensing concepts supporting bio-analytics applications transversal to the main biomedical stream. The advancements concerning enabling biomaterials and related novel biosensing concepts involving optogenetics are reviewed with particular focus on the use of engineered cells for cell-based sensing platforms and the available toolbox (from mere actuators and reporters to novel multifunctional opto-chemogenetic tools) for optogenetic-enabled real-time cellular diagnostics and biosensor development. The key advantages of these modified cell-based biosensors concern both significantly faster (minutes instead of hours) and higher sensitivity detection of low concentrations of bioactive/toxic analytes (below the threshold concentrations in classical cellular sensors) as well as improved standardization as warranted by unified analytic platforms. These novel multimodal functional electro-optical label-free assays are reviewed among the key elements for optogenetic-based biosensing standardization. This focused review is a potential guide for materials researchers interested in biosensing based on light-responsive biomaterials and related analytic tools.
Keywords: cell dynamics; cell-based biosensors; optogenetic (light-responsive) biomaterials; optogenetic control; time-lapse multiparametric assays.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

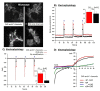
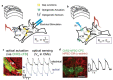

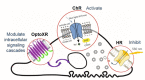
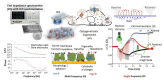
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

