Postharvest Drying Techniques Regulate Secondary Metabolites and Anti-Neuroinflammatory Activities of Ganoderma lucidum
- PMID: 34361637
- PMCID: PMC8347575
- DOI: 10.3390/molecules26154484
Postharvest Drying Techniques Regulate Secondary Metabolites and Anti-Neuroinflammatory Activities of Ganoderma lucidum
Abstract
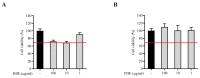
Ganoderma lucidum extract is a potent traditional remedy for curing various ailments. Drying is the most important postharvest step during the processing of Ganoderma lucidum. The drying process mainly involves heat (36 h at 60 °C) and freeze-drying (36 h at -80 °C). We investigated the effects of different postharvest drying protocols on the metabolites profiling of Ganoderma lucidum using GC-MS, followed by an investigation of the anti-neuroinflammatory potential in LPS-treated BV2 microglial cells. A total of 109 primary metabolites were detected from heat and freeze-dried samples. Primary metabolite profiling showed higher levels of amino acids (17.4%) and monosaccharides (8.8%) in the heat-dried extracts, whereas high levels of organic acids (64.1%) were present in the freeze-dried samples. The enzymatic activity, such as ATP-citrate synthase, pyruvate kinase, glyceraldehyde-3-phosphatase dehydrogenase, glutamine synthase, fructose-bisphosphate aldolase, and D-3-phosphoglycerate dehydrogenase, related to the reverse tricarboxylic acid cycle were significantly high in the heat-dried samples. We also observed a decreased phosphorylation level of the MAP kinase (Erk1/2, p38, and JNK) and NF-κB subunit p65 in the heat-dried samples of the BV2 microglia cells. The current study suggests that heat drying improves the production of ganoderic acids by the upregulation of TCA-related pathways, which, in turn, gives a significant reduction in the inflammatory response of LPS-induced BV2 cells. This may be attributed to the inhibition of NF-κB and MAP kinase signaling pathways in cells treated with heat-dried extracts.
Keywords: BV2 cancer cells; Ganoderma lucidum; LPS-induced inflammation; MAPK; ganoderic acid; neuro-degradation.
Conflict of interest statement
The authors declare no conflict of interest with respect to the authorship and/or publication of this article.
Figures




References
-
- Desa M., Nurlaila W., Mohammad M., Fudholi A. Review of drying technology of Figure. Trends Food Sci. Technol. 2019;88:93–103. doi: 10.1016/j.tifs.2019.03.018. - DOI
-
- Adali M.B., Barresi A.A., Boccardo G., Pisano R. Spray Freeze-Drying as a Solution to Continuous Manufacturing of Pharmaceutical Products in Bulk. Processes. 2020;8:709. doi: 10.3390/pr8060709. - DOI
-
- Prosapio V., Norton I. Influence of osmotic dehydration pre-treatment on oven drying and freeze drying performance. LWT. 2017;80:401–408. doi: 10.1016/j.lwt.2017.03.012. - DOI
-
- Marques L.G., Prado M.M., Freire J.T. Rehydration characteristics of freeze-dried tropical fruits. LWT. 2009;42:1232–1237. doi: 10.1016/j.lwt.2009.02.012. - DOI
-
- Barimah J., Yanney P., Laryea D., Quarcoo C. Effect of Drying Methods on Phytochemicals, Antioxidant Activity and Total Phenolic Content of Dandelion Leaves. Am. J. Food Nutr. 2017;5:136–141.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

