Noscapine Prevents Rotenone-Induced Neurotoxicity: Involvement of Oxidative Stress, Neuroinflammation and Autophagy Pathways
- PMID: 34361780
- PMCID: PMC8348109
- DOI: 10.3390/molecules26154627
Noscapine Prevents Rotenone-Induced Neurotoxicity: Involvement of Oxidative Stress, Neuroinflammation and Autophagy Pathways
Abstract
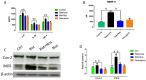
Parkinson's disease is characterized by the loss of dopaminergic neurons in substantia nigra pars compacta (SNpc) and the resultant loss of dopamine in the striatum. Various studies have shown that oxidative stress and neuroinflammation plays a major role in PD progression. In addition, the autophagy lysosome pathway (ALP) plays an important role in the degradation of aggregated proteins, abnormal cytoplasmic organelles and proteins for intracellular homeostasis. Dysfunction of ALP results in the accumulation of α-synuclein and the loss of dopaminergic neurons in PD. Thus, modulating ALP is becoming an appealing therapeutic intervention. In our current study, we wanted to evaluate the neuroprotective potency of noscapine in a rotenone-induced PD rat model. Rats were administered rotenone injections (2.5 mg/kg, i.p.,) daily followed by noscapine (10 mg/kg, i.p.,) for four weeks. Noscapine, an iso-qinulinin alkaloid found naturally in the Papaveraceae family, has traditionally been used in the treatment of cancer, stroke and fibrosis. However, the neuroprotective potency of noscapine has not been analyzed. Our study showed that administration of noscapine decreased the upregulation of pro-inflammatory factors, oxidative stress, and α-synuclein expression with a significant increase in antioxidant enzymes. In addition, noscapine prevented rotenone-induced activation of microglia and astrocytes. These neuroprotective mechanisms resulted in a decrease in dopaminergic neuron loss in SNpc and neuronal fibers in the striatum. Further, noscapine administration enhanced the mTOR-mediated p70S6K pathway as well as inhibited apoptosis. In addition to these mechanisms, noscapine prevented a rotenone-mediated increase in lysosomal degradation, resulting in a decrease in α-synuclein aggregation. However, further studies are needed to further develop noscapine as a potential therapeutic candidate for PD treatment.
Keywords: Parkinson’s disease; autophagy; inflammation; noscapine; oxidative stress; rotenone.
Conflict of interest statement
The authors declare no conflict of interest. Funders have no role in project design, investigation and report writing.
Figures






References
-
- Martinez-Martin P., Rodriguez-Blazquez C., Paz S., Forjaz M.J., Frades-Payo B., Cubo E., De Pedro-Cuesta J., Lizan L., ELEP Group Parkinson Symptoms and Health Related Quality of Life as Predictors of Costs: A Longitudinal Observational Study with Linear Mixed Model Analysis. PLoS ONE. 2015;10:e0145310. doi: 10.1371/journal.pone.0145310. - DOI - PMC - PubMed
-
- Dorsey E.R., Elbaz A., Nichols E., Abd-Allah F., Abdelalim A., Adsuar J.C., Ansha M.G., Brayne C., Choi J.-Y.J., Collado-Mateo D., et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

