Analysis of Organophosphorus-Based Nerve Agent Degradation Products by Gas Chromatography-Mass Spectrometry (GC-MS): Current Derivatization Reactions in the Analytical Chemist's Toolbox
- PMID: 34361784
- PMCID: PMC8348239
- DOI: 10.3390/molecules26154631
Analysis of Organophosphorus-Based Nerve Agent Degradation Products by Gas Chromatography-Mass Spectrometry (GC-MS): Current Derivatization Reactions in the Analytical Chemist's Toolbox
Abstract
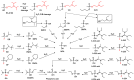
The field of gas chromatography-mass spectrometry (GC-MS) in the analysis of chemical warfare agents (CWAs), specifically those involving the organophosphorus-based nerve agents (OPNAs), is a continually evolving and dynamic area of research. The ever-present interest in this field within analytical chemistry is driven by the constant threat posed by these lethal CWAs, highlighted by their use during the Tokyo subway attack in 1995, their deliberate use on civilians in Syria in 2013, and their use in the poisoning of Sergei and Yulia Skripal in Great Britain in 2018 and Alexei Navalny in 2020. These events coupled with their potential for mass destruction only serve to stress the importance of developing methods for their rapid and unambiguous detection. Although the direct detection of OPNAs is possible by GC-MS, in most instances, the analytical chemist must rely on the detection of the products arising from their degradation. To this end, derivatization reactions mainly in the form of silylations and alkylations employing a vast array of reagents have played a pivotal role in the efficient detection of these products that can be used retrospectively to identify the original OPNA.
Keywords: GC-MS; Novichoks; chemical warfare agents; derivatization; methylation; nerve agents; sarin; silylation.
Conflict of interest statement
The authors declare no conflict of interest. This document (LLNL-JRNL-823194) was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor Lawrence Livermore National Security, LLC, nor any of their employees makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or Lawrence Livermore National Security, LLC. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or Lawrence Livermore National Security, LLC, and shall not be used for advertising or product endorsement purposes.
Figures


References
-
- Haines D.D., Fox S.C. Acute and long-term impact of chemical weapons: Lessons from the Iran-Iraq war. Forensic Sci. Rev. 2014;26:97–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

