Absorption Coefficients of Phenolic Structures in Different Solvents Routinely Used for Experiments
- PMID: 34361808
- PMCID: PMC8348453
- DOI: 10.3390/molecules26154656
Absorption Coefficients of Phenolic Structures in Different Solvents Routinely Used for Experiments
Abstract
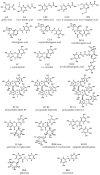
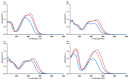
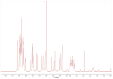
Phenolic structures are of great interest due to their antioxidant properties and various postulated benefits on human health. However, the quantification of these structures in fruits and vegetables, as well as in vivo or in vitro experiments, is demanding, as relevant concentrations are often low, causing problems in exactly weighing the respective amounts. Nevertheless, the determination of used concentrations is often a prerequisite for accurate results. A possibility to quantify polyphenol is the use of UV/vis spectroscopy. Therefore, the absorption coefficients of selected phenolic structures were determined in three different solvents relevant for polyphenol research (water/methanol (50/50, v/v), water, and phosphate buffer at pH 7.5). To confirm the values based on weight and to avoid errors due to impurities, hygroscopic effects, and inadequate balance care, the mass concentrations were additionally determined by quantitative NMR (q-NMR). The coefficients presented in this article can help to quickly and easily determine accurate concentrations in a laboratory routine without wasting the often-precious standard compounds.
Keywords: absorption coefficient; anthocyanins; polyphenols; q-NMR.
Conflict of interest statement
All authors declare that there is no conflict of interest.
Figures


Similar articles
-
Water-soluble vitamins.J AOAC Int. 2006 Jan-Feb;89(1):285-8. J AOAC Int. 2006. PMID: 16512258
-
Phytochemical Characterization of Five Edible Purple-Reddish Vegetables: Anthocyanins, Flavonoids, and Phenolic Acid Derivatives.Molecules. 2019 Apr 18;24(8):1536. doi: 10.3390/molecules24081536. Molecules. 2019. PMID: 31003505 Free PMC article.
-
Some analytical assays for the determination of bioactivity of exotic fruits.Phytochem Anal. 2010 Jul-Aug;21(4):355-62. doi: 10.1002/pca.1207. Phytochem Anal. 2010. PMID: 20183860
-
Anthocyanin Profiling Using UV-Vis Spectroscopy and Liquid Chromatography Mass Spectrometry.J AOAC Int. 2020 Jan 1;103(1):23-39. doi: 10.5740/jaoacint.19-0201. J AOAC Int. 2020. PMID: 31462350 Review.
-
NMR spectroscopy: a powerful tool for the analysis of polyphenols in extra virgin olive oil.J Sci Food Agric. 2020 Mar 30;100(5):1842-1851. doi: 10.1002/jsfa.10173. Epub 2019 Dec 28. J Sci Food Agric. 2020. PMID: 31802495 Review.
Cited by
-
Applying Isothermal Titration Calorimetry and Saturation Transfer Difference-NMR to Study the Mode of Interaction of Flavan-3-ols with α-Amylase to Understand Their Impact on Starch Hydrolysis.J Agric Food Chem. 2025 Apr 16;73(15):9047-9061. doi: 10.1021/acs.jafc.4c13178. Epub 2025 Apr 4. J Agric Food Chem. 2025. PMID: 40184499 Free PMC article.
-
Nano-Liposomal Carrier as Promising Dermal Delivery Platform for Fumaria officinalis L. Bioactives.Pharmaceutics. 2025 Jun 14;17(6):782. doi: 10.3390/pharmaceutics17060782. Pharmaceutics. 2025. PMID: 40574093 Free PMC article.
-
Liposomal Encapsulation of Carob (Ceratonia siliqua L.) Pulp Extract: Design, Characterization, and Controlled Release Assessment.Pharmaceutics. 2025 Jun 13;17(6):776. doi: 10.3390/pharmaceutics17060776. Pharmaceutics. 2025. PMID: 40574089 Free PMC article.
-
Neutral and Pectic Heteropolysaccharides Isolated from Opuntia joconostle Mucilage: Composition, Molecular Dimensions and Prebiotic Potential.Int J Mol Sci. 2023 Feb 6;24(4):3208. doi: 10.3390/ijms24043208. Int J Mol Sci. 2023. PMID: 36834619 Free PMC article.
-
Design and Characterization of Liposomal-Based Carriers for the Encapsulation of Rosa canina Fruit Extract: In Vitro Gastrointestinal Release Behavior.Plants (Basel). 2024 Sep 18;13(18):2608. doi: 10.3390/plants13182608. Plants (Basel). 2024. PMID: 39339584 Free PMC article.
References
-
- Jakobek L., García-Villalba R., Tomás-Barberán F.A. Polyphenolic characterisation of old local apple varieties from Southeastern European region. J. Food Compost. Anal. 2013;31:199–211. doi: 10.1016/j.jfca.2013.05.012. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

