Recent Advances in Biopolymer-Based Dye Removal Technologies
- PMID: 34361855
- PMCID: PMC8347927
- DOI: 10.3390/molecules26154697
Recent Advances in Biopolymer-Based Dye Removal Technologies
Abstract
Synthetic dyes have become an integral part of many industries such as textiles, tannin and even food and pharmaceuticals. Industrial dye effluents from various dye utilizing industries are considered harmful to the environment and human health due to their intense color, toxicity and carcinogenic nature. To mitigate environmental and public health related issues, different techniques of dye remediation have been widely investigated. However, efficient and cost-effective methods of dye removal have not been fully established yet. This paper highlights and presents a review of recent literature on the utilization of the most widely available biopolymers, specifically, cellulose, chitin and chitosan-based products for dye removal. The focus has been limited to the three most widely explored technologies: adsorption, advanced oxidation processes and membrane filtration. Due to their high efficiency in dye removal coupled with environmental benignity, scalability, low cost and non-toxicity, biopolymer-based dye removal technologies have the potential to become sustainable alternatives for the remediation of industrial dye effluents as well as contaminated water bodies.
Keywords: adsorption; advanced oxidation processes; biopolymers; cellulose; chitin; chitosan; dye removal; membrane filtration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
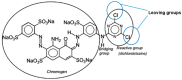
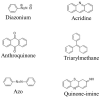

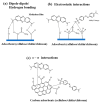

References
-
- Hussain S., Khan N., Gul S., Khan S., Khan H. Contamination of Water Resources by Food Dyes and Its Removal Technologies. In: Eyvaz M., Yüksel E., editors. Water Chemistry. Intech Open; London, UK: 2020.
-
- Acharya S. Master’s Thesis. Texas Tech University; Lubbock, TX, USA: 2012. Cationization of Cotton Fabric for Improved Dye Uptake.
-
- Siva R. Status of natural dyes and dye-yielding plants in India. Curr. Sci. 2007;92:916–925.
-
- Saini R.D. Textile Organic Dyes: Polluting effects and Elimination Methods from Textile Waste Water. Int. J. Chem. Eng. Res. 2017;9:975–6442.
-
- Cañamares M.V., Reagan D.A., Lombardi J.R., Leona M. TLC-SERS of mauve, the first synthetic dye. J. Raman Spectrosc. 2014;45:1147–1152. doi: 10.1002/jrs.4508. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

