Microbial Diversity of Terrestrial Geothermal Springs in Armenia and Nagorno-Karabakh: A Review
- PMID: 34361908
- PMCID: PMC8307006
- DOI: 10.3390/microorganisms9071473
Microbial Diversity of Terrestrial Geothermal Springs in Armenia and Nagorno-Karabakh: A Review
Abstract
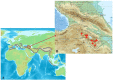
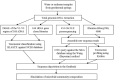
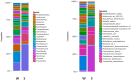
The microbial diversity of high-altitude geothermal springs has been recently assessed to explore their biotechnological potential. However, little is known regarding the microbiota of similar ecosystems located on the Armenian Highland. This review summarizes the known information on the microbiota of nine high-altitude mineralized geothermal springs (temperature range 25.8-70 °C and pH range 6.0-7.5) in Armenia and Nagorno-Karabakh. All these geothermal springs are at altitudes ranging from 960-2090 m above sea level and are located on the Alpide (Alpine-Himalayan) orogenic belt, a seismically active region. A mixed-cation mixed-anion composition, with total mineralization of 0.5 mg/L, has been identified for these thermal springs. The taxonomic diversity of hot spring microbiomes has been examined using culture-independent approaches, including denaturing gradient gel electrophoresis (DGGE), 16S rRNA gene library construction, 454 pyrosequencing, and Illumina HiSeq. The bacterial phyla Proteobacteria, Bacteroidetes, Cyanobacteria, and Firmicutes are the predominant life forms in the studied springs. Archaea mainly include the phyla Euryarchaeota, Crenarchaeota, and Thaumarchaeota, and comprise less than 1% of the prokaryotic community. Comparison of microbial diversity in springs from Karvachar with that described for other terrestrial hot springs revealed that Proteobacteria, Bacteroidetes, Actinobacteria, and Deinococcus-Thermus are the common bacterial groups in terrestrial hot springs. Contemporaneously, specific bacterial and archaeal taxa were observed in different springs. Evaluation of the carbon, sulfur, and nitrogen metabolism in these hot spring communities has revealed diversity in terms of metabolic activity. Temperature seems to be an important factor in shaping the microbial communities of these springs. Overall, the diversity and richness of the microbiota are negatively affected by increasing temperature. Other abiotic factors, including pH, mineralization, and geological history, also impact the structure and function of the microbial community. More than 130 bacterial and archaeal strains (Bacillus, Geobacillus, Parageobacillus, Anoxybacillus, Paenibacillus, Brevibacillus Aeribacillus, Ureibacillus, Thermoactinomyces, Sporosarcina, Thermus, Rhodobacter, Thiospirillum, Thiocapsa, Rhodopseudomonas, Methylocaldum, Desulfomicrobium, Desulfovibrio, Treponema, Arcobacter, Nitropspira, and Methanoculleus) have been reported, some of which may be representative of novel species (sharing 91-97% sequence identity with their closest matches in GenBank) and producers of thermozymes and biomolecules with potential biotechnological applications. Whole-genome shotgun sequencing of T. scotoductus K1, as well as of the potentially new Treponema sp. J25 and Anoxybacillus sp. K1, were performed. Most of the phyla identified by 16S rRNA were also identified using metagenomic approaches. Detailed characterization of thermophilic isolates indicate the potential of the studied springs as a source of biotechnologically valuable microbes and biomolecules.
Keywords: bioactive compounds; biotechnology; culture-dependent and -independent techniques; geothermal springs; microbial diversity; thermophiles; thermozymes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hreggvidsson G.O., Petursdottir S.K., Bjornsdottir S.H., Fridjonsson O.H. Microbial speciation in the geothermal ecosystem. In: Stan H., Fendrihan L.S., editors. Adaption of Microbial Life to Environmental Extremes: Novel Research Results and Application. Springer; Vienna, Austria: 2012. pp. 37–68.
-
- Deepika M., Satyanarayana T. Diversity of Hot Environments and Thermophilic Microbes. In: Satyanarayana T., Littlechild J., Kawarabayasi Y., editors. Thermophilic Microbes in Environmental and Industrial Biotechnology Biotechnology of Thermophiles. Springer; Dordrecht, The Netherlands: 2013. pp. 3–60.
-
- Stan-Lotter H., Fendrihan S. Adaption of Microbial Life to Environmental Extremes. Springer; Vienna, Austria: 2012.
Publication types
LinkOut - more resources
Full Text Sources

