Liposomal Inhalation after Tracheostomy-A Randomized Controlled Trial
- PMID: 34362096
- PMCID: PMC8348021
- DOI: 10.3390/jcm10153312
Liposomal Inhalation after Tracheostomy-A Randomized Controlled Trial
Abstract
Background: Tracheostomy is a common procedure in critical care. The aim of this study was to evaluate the application of a liposomal inhalation compared to standard physiologic saline (SPS) inhalation on basis of objective and subjective parameters of airway inflammation.
Methods: We evaluated in this two-armed, double-blinded and randomized control group study the effect of liposomal compared with SPS inhalation in newly tracheotomized patients. The primary endpoint was defined as trend of tracheobronchial IL-6 secretion at day 1 compared to day 10. Further objective and subjective parameter were evaluated.
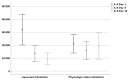
Results: Fifty patients were randomized in each arm. Tracheal IL-6 levels decreased significantly only after liposomal inhalation. Both inhalative agents seem to have an effect on the respiratory impairment after tracheostomy. Subjective patient impairment was reduced significantly from day 1 to day 10 after tracheostomy with liposomal inhalation.
Conclusions: Liposomal inhalation demonstrated an advantage over SPS inhalation in newly tracheotomized patients.
Keywords: inhalation; interleukin; liposomes; physiological saline solution; tracheostomy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Pignatti P., Balestrino A., Herr C., Bals R., Moretto D., Corradi M., Alinovi R., Delmastro M., Vogelmeier C., Nava S., et al. Tracheostomy and related host-pathogen interaction are associated with airway inflammation as characterized by tracheal aspirate analysis. Respir. Med. 2009;103:201–208. doi: 10.1016/j.rmed.2008.09.008. - DOI - PubMed
-
- Hill N.S. Neuromuscular disease in respiratory and critical care medicine. Respir. Care. 2006;51:1065–1071. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical