Are Patients with Psoriasis and Psoriatic Arthritis Undertreated? A Population-Based Study from Southern Italy
- PMID: 34362214
- PMCID: PMC8348176
- DOI: 10.3390/jcm10153431
Are Patients with Psoriasis and Psoriatic Arthritis Undertreated? A Population-Based Study from Southern Italy
Abstract
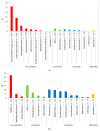
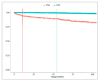
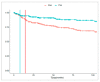
This study aimed to explore the pattern of use of different treatment lines in psoriasis (PsO) and psoriatic arthritis (PsA) patients from Southern Italy. A retrospective cohort study was performed during the years 2010-2018 using data from the Caserta Local Health Unit (LHU) claims database. All of the PsO or PsA patients were identified. The proportion of PsO/PsA patients untreated or treated with ≥1 drug classes (i.e., non-disease-modifying antirheumatic drugs (non-DMARDs), conventional synthetic DMARDs (csDMARDs), biological drugs (bDMARDs) or targeted synthetic small molecules (tsDMARDs)) was calculated in the years 2016-2018. Among the bDMARD users, the median times from the first registered PsO/PsA diagnosis/from the first csDMARD to the first bDMARD were calculated. Overall, 10,296 (1.1%) and 1724 (0.2%) PsO and PsA patients were identified. More than half of the PsO patients (N = 5301; 51.6%) and 15% of the PsA patients (N = 251) were not treated with any drug. A very low proportion of PsO patients (N = 121; 1.2%) received csDMARDs/bDMARDs dispensing. Instead, 538 (32.2%) PsA patients were treated with bDMARDs. The median times from the first diagnosis to the first bDMARD dispensing were 54.0 (Q1-Q3: 30.5-72.2) and 13.3 (Q1-Q3: 3.1-43.9) months in the PsO and PsA patients, respectively. The median time from the first csDMARD to the first bDMARD dispensing was shorter in the PsO [9.2 months (Q1-Q3: 5.5-30.0)] than in the PsA [14.5 months (Q1-Q3: 8.6-33.5)] patients. A potential undertreatment of PsO (much less for PsA) in an LHU from Southern Italy, with a particularly low use of more recently marketed drugs, such as biological ones, was shown.
Keywords: biological drugs; psoriasis; psoriatic arthritis; undertreatment.
Conflict of interest statement
G.T. has served on advisory boards/seminars funded by SANOFI, Eli Lilly, AstraZeneca, Abbvie, Servier, Mylan, Gilead and Amgen; he was the scientific director of a II level Master on pharmacovigilance, pharmacoepidemiology and real-world evidence, which has received non-conditional contributions from various pharmaceutical companies; he coordinates a pharmacoepidemiology team at the University of Messina, which has received funding for conducting observational studies from various pharmaceutical companies (Boehringer Ingelheim, Daichii Sankyo, PTC Pharmaceuticals). He is also chief of the academic spin-off “INSPIRE srl”, which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions, Pharmo Institute N.V.). Y.I. is the CEO of the academic spin-off “INSPIRE srl” of the University of Messina, which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions, Pharmo Institute N.V.). C.G. has received consultation fees and/or grants for research projects, advisory panels and giving educational lectures from Pfizer, Abbvie, Janssen-Cilag, Novartis, LEO Pharma, Bayer, Ely Lilly, Celgene, Merck Sharp & Dome, Sanofi, Amgen and Almirall. He is also a member of the academic spin-off “INSPIRE srl”, which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions, Pharmo Institute N.V.). V.I. (Valentina Isgrò), V.I. (Valentina Ientile) and M.T. declare that they have no conflicts of interest.
Figures


References
-
- Pezzolo E., Cazzaniga S., Colombo P., Chatenoud L., Naldi L. Psoriasis Incidence and Lifetime Prevalence: Suggestion for a Higher Mortality Rate in Older Age-classes among Psoriatic Patients Compared to the General Population in Italy. Acta Derm. Venereol. 2019;99:400–403. doi: 10.2340/00015555-3130. - DOI - PubMed
-
- Naldi L., Pini P., Girolomoni G., Gestione Clinica della Psoriasi Pacini Editore Medicina. [(accessed on 1 November 2020)]; Available online: https://www.pacinimedicina.it/wp-content/uploads/Layout_4B_Girolomoni.pdf.
-
- Prey S., Paul C., Bronsard V., Puzenat E., Gourraud P.-A., Aractingi S., Aubin F., Bagot M., Cribier B., Joly P., et al. Assessment of risk of psoriatic arthritis in patients with plaque psoriasis: A systematic review of the literature. J. Eur. Acad. Dermatol. Venereol. 2010;24(Suppl. 2):31–35. doi: 10.1111/j.1468-3083.2009.03565.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

