Inhibition of autophagy by CRMP2-derived peptide ST2-104 (R9-CBD3) via a CaMKKβ/AMPK/mTOR pathway contributes to ischemic postconditioning-induced neuroprotection against cerebral ischemia-reperfusion injury
- PMID: 34362425
- PMCID: PMC8344221
- DOI: 10.1186/s13041-021-00836-0
Inhibition of autophagy by CRMP2-derived peptide ST2-104 (R9-CBD3) via a CaMKKβ/AMPK/mTOR pathway contributes to ischemic postconditioning-induced neuroprotection against cerebral ischemia-reperfusion injury
Abstract
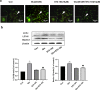
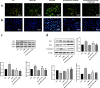
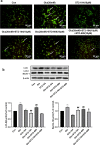
Cerebral ischemia, a common cerebrovascular disease, is characterized by functional deficits and apoptotic cell death. Autophagy, a type of programmed cell death, plays critical roles in controlling neuronal damage and metabolic homeostasis, and has been inextricably linked to cerebral ischemia. We previously identified a short peptide aptamer from collapsin response mediator protein 2 (CRMP2), designated the Ca2+ channel-binding domain 3 (CBD3) peptide, that conferred protection against excitotoxicity and traumatic brain injury. ST2-104, a nona-arginine (R9)-fused CBD3 peptide, exerted beneficial effects on neuropathic pain and was neuroprotective in a model of Alzheimer's disease; however, the effect of ST2-104 on cerebral ischemia and its mechanism of action have not been studied. In this study, we modeled cerebral ischemia-reperfusion injury in rats with the middle cerebral artery occlusion (MCAO) as well as challenged SH-SY5Y neuroblastoma cells with glutamate to induce toxicity to interrogate the effects of ST2-104 on autophagy following ischemic/excitotoxic insults. ST2-104 reduced the infarct volume and improved the neurological score of rats subjected to MCAO. ST2-104 protected SH-SY5Y cells from death following glutamate exposure via blunting apoptosis and autophagy as well as limiting excessive calcium entry. 3-Methyladenine (3-MA), an inhibitor of autophagy, promoted the effects of ST2-104 in inhibiting apoptosis triggered by glutamate while rapamycin, an activator of autophagy, failed to do so. ST2-104 peptide reversed glutamate-induced apoptosis via inhibiting Ca2+/CaM-dependent protein kinase kinase β (CaMKKβ)-mediated autophagy, which was partly enhanced by STO-609 (an inhibitor of CaMKKβ). ST2-104 attenuated neuronal apoptosis by inhibiting autophagy through CaMKKβ/AMPK/mTOR pathway. Our results suggest that the neuroprotective effect of ST2-104 are due to actions on the crosstalk between apoptosis and autophagy via the CaMKKβ/AMPK/mTOR signaling pathway. The findings present novel insights into the potential neuroprotection of ST2-104 in cerebral ischemia.
Keywords: Apoptosis; Autophagy; CRMP2; CaMKKβ/AMPK/mTOR pathway; Cerebral ischemia injury; Glutamate.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Aβ25-35-induced autophagy and apoptosis are prevented by the CRMP2-derived peptide ST2-104 (R9-CBD3) via a CaMKKβ/AMPK/mTOR signaling hub.PLoS One. 2024 Sep 26;19(9):e0309794. doi: 10.1371/journal.pone.0309794. eCollection 2024. PLoS One. 2024. PMID: 39325788 Free PMC article.
-
CRMP2-derived peptide ST2-104 (R9-CBD3) protects SH-SY5Y neuroblastoma cells against Aβ25-35-induced neurotoxicity by inhibiting the pCRMP2/NMDAR2B signaling pathway.Chem Biol Interact. 2019 May 25;305:28-39. doi: 10.1016/j.cbi.2019.03.005. Epub 2019 Mar 12. Chem Biol Interact. 2019. PMID: 30871964
-
Propofol inhibited autophagy through Ca2+/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron injury.Mol Med. 2018 Nov 23;24(1):58. doi: 10.1186/s10020-018-0054-1. Mol Med. 2018. PMID: 30470173 Free PMC article.
-
Challenging the catechism of therapeutics for chronic neuropathic pain: Targeting CaV2.2 interactions with CRMP2 peptides.Neurosci Lett. 2013 Dec 17;557 Pt A(0 0):27-36. doi: 10.1016/j.neulet.2013.06.057. Epub 2013 Jul 3. Neurosci Lett. 2013. PMID: 23831344 Free PMC article. Review.
-
Targeting intracellular autophagic process for the treatment of post-stroke ischemia/reperfusion injury.Animal Model Exp Med. 2025 Mar;8(3):389-404. doi: 10.1002/ame2.12528. Epub 2025 Feb 5. Animal Model Exp Med. 2025. PMID: 39908171 Free PMC article. Review.
Cited by
-
EGCG protects the mouse brain against cerebral ischemia/reperfusion injury by suppressing autophagy via the AKT/AMPK/mTOR phosphorylation pathway.Front Pharmacol. 2022 Sep 6;13:921394. doi: 10.3389/fphar.2022.921394. eCollection 2022. Front Pharmacol. 2022. PMID: 36147330 Free PMC article.
-
Dysregulation of mTOR Signaling after Brain Ischemia.Int J Mol Sci. 2022 Mar 4;23(5):2814. doi: 10.3390/ijms23052814. Int J Mol Sci. 2022. PMID: 35269956 Free PMC article. Review.
-
Neuroprotective Peptides and New Strategies for Ischemic Stroke Drug Discoveries.Genes (Basel). 2023 Apr 22;14(5):953. doi: 10.3390/genes14050953. Genes (Basel). 2023. PMID: 37239313 Free PMC article. Review.
-
Targeting AMP-activated protein kinase in sepsis.Front Endocrinol (Lausanne). 2024 Oct 14;15:1452993. doi: 10.3389/fendo.2024.1452993. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39469575 Free PMC article. Review.
-
The underlying mechanism of calcium toxicity-induced autophagic cell death and lysosomal degradation in early stage of cerebral ischemia.Anat Cell Biol. 2024 Jun 30;57(2):155-162. doi: 10.5115/acb.24.003. Epub 2024 Apr 29. Anat Cell Biol. 2024. PMID: 38680098 Free PMC article. Review.
References
-
- Gao Y, Wen LL, Yang X, Wang J, Feng J. Pathological mechanism of focal cerebral ischemia and reperfusion injuries in mice. J Biol Regul Homeost Agents. 2019;33(5):1507–1513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

