Novel TLR4 adjuvant elicits protection against homologous and heterologous Influenza A infection
- PMID: 34362603
- PMCID: PMC8744187
- DOI: 10.1016/j.vaccine.2021.06.085
Novel TLR4 adjuvant elicits protection against homologous and heterologous Influenza A infection
Abstract
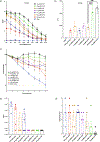
Influenza A virus (IAV) is a leading cause of respiratory disease worldwide often resulting in hospitalization or death. In this study, TLR4 immunostimulatory molecules, Bacterial Enzymatic Combinatorial Chemistry (BECC) 438 and BECC470 were found to be superior IAV vaccine adjuvants when compared to the classic adjuvant alhydrogel (alum) and Phosphorylated Hexa-Acyl Disaccharide (PHAD), a synthetic TLR4 agonist. BECC molecules allow for antigen sparing of a recombinant HA (rHA) protein, elicit a more balanced IgG1/IgG2a response, and were protective in a prime only dosing schedule. Importantly, BECC molecules afford protection from a heterologous IAV strain demonstrating that a cross-protective influenza vaccine is possible when the antigen is effectively adjuvanted.
Keywords: Adjuvant; Cross-protection; Influenza; TLR4 agonist; Vaccine.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Molinari NA, et al., The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine, 2007. 25(27): p. 5086–96. - PubMed
-
- Reid AH and Taubenberger JK, The 1918 flu and other influenza pandemics: “over there” and back again. Lab Invest, 1999. 79(2): p. 95–101. - PubMed
-
- Treanor J, Influenza vaccine--outmaneuvering antigenic shift and drift. N Engl J Med, 2004. 350(3): p. 218–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

