Longitudinal tracking of neuronal mitochondria delineates PINK1/Parkin-dependent mechanisms of mitochondrial recycling and degradation
- PMID: 34362731
- PMCID: PMC8346224
- DOI: 10.1126/sciadv.abf6580
Longitudinal tracking of neuronal mitochondria delineates PINK1/Parkin-dependent mechanisms of mitochondrial recycling and degradation
Abstract
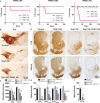
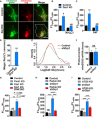
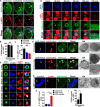
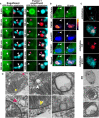
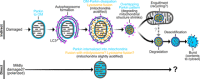
Altered mitochondrial quality control and dynamics may contribute to neurodegenerative diseases, including Parkinson's disease, but we understand little about these processes in neurons. We combined time-lapse microscopy and correlative light and electron microscopy to track individual mitochondria in neurons lacking the fission-promoting protein dynamin-related protein 1 (Drp1) and delineate the kinetics of PINK1-dependent pathways of mitochondrial quality control. Depolarized mitochondria recruit Parkin to the outer mitochondrial membrane, triggering autophagosome formation, rapid lysosomal fusion, and Parkin redistribution. Unexpectedly, these mitolysosomes are dynamic and persist for hours. Some are engulfed by healthy mitochondria, and others are deacidified before bursting. In other cases, Parkin is directly recruited to the matrix of polarized mitochondria. Loss of PINK1 blocks Parkin recruitment, causes LC3 accumulation within mitochondria, and exacerbates Drp1KO toxicity to dopamine neurons. These results define a distinct neuronal mitochondrial life cycle, revealing potential mechanisms of mitochondrial recycling and signaling relevant to neurodegeneration.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



References
-
- Nakamura K., Nemani V. M., Azarbal F., Skibinski G., Levy J. M., Egami K., Munishkina L., Zhang J., Gardner B., Wakabayashi J., Sesaki H., Cheng Y., Finkbeiner S., Nussbaum R. L., Masliah E., Edwards R. H., Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J. Biol. Chem. 286, 20710–20726 (2011). - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

