Endoscopy in patients on antiplatelet or anticoagulant therapy: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guideline update
- PMID: 34362780
- PMCID: PMC8355884
- DOI: 10.1136/gutjnl-2021-325184
Endoscopy in patients on antiplatelet or anticoagulant therapy: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guideline update
Abstract
This is a collaboration between the British Society of Gastroenterology (BSG) and the European Society of Gastrointestinal Endoscopy (ESGE), and is a scheduled update of their 2016 guideline on endoscopy in patients on antiplatelet or anticoagulant therapy. The guideline development committee included representatives from the British Society of Haematology, the British Cardiovascular Intervention Society, and two patient representatives from the charities Anticoagulation UK and Thrombosis UK, as well as gastroenterologists. The process conformed to AGREE II principles and the quality of evidence and strength of recommendations were derived using GRADE methodology. Prior to submission for publication, consultation was made with all member societies of ESGE, including BSG. Evidence-based revisions have been made to the risk categories for endoscopic procedures, and to the categories for risks of thrombosis. In particular a more detailed risk analysis for atrial fibrillation has been employed, and the recommendations for direct oral anticoagulants have been strengthened in light of trial data published since the previous version. A section has been added on the management of patients presenting with acute GI haemorrhage. Important patient considerations are highlighted. Recommendations are based on the risk balance between thrombosis and haemorrhage in given situations.
Keywords: endoscopic procedures.
© 2021 European Society of Gastrointestinal Endoscopy, British Society of Gastroenterology, the Author(s) or their employer(s).
Conflict of interest statement
Competing interests: FR has received speaker fees from Bristol-Meyers Squibb, Pfizer and Boehringer Ingelheim. Dr Alikhan has received fees from Alexion, Bayer, Boehringer Ingelheim, Bristol-Meyers Squibb, Daiichi, Pfizer and Portola. WL has received speaker fees from Sanofi Aventis and Leo Pharma, speaker fees and advisory board fees from Bayer, Daichii Sankyo, Pfizer and Boehringer Ingelheim, and support to attend a scientific meeting from Boehringer Ingelheim. JEV-H has received departmental research grant support from Cook Medical and Abbott, lecture fees from Medtronics and Cook Medical, and consultancy fees from Boston Scientific and Olympus.
Figures

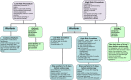
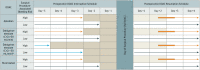
References
-
- Veitch AM, Vanbiervliet G, Gershlick AH, et al. . Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of gastroenterology (Bsg) and European Society of gastrointestinal endoscopy (ESGE) guidelines. Gut 2016;65:374–89. 10.1136/gutjnl-2015-311110 - DOI - PMC - PubMed
-
- Veitch AM, Vanbiervliet G, Gershlick AH, et al. . Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of gastroenterology (Bsg) and European Society of gastrointestinal endoscopy (ESGE) guidelines. Endoscopy 2016;48:385–402. 10.1055/s-0042-102652 - DOI - PubMed
-
- AGREE Next Steps Consortium . The agree II instrument (electronic version), 2009. Available: http://www.agreetrust.org [Accessed May 2021].
-
- Guyatt GH, Oxman AD, Vist GE, et al. . Grade: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
