Derivation of a prototype asthma attack risk scale centred on blood eosinophils and exhaled nitric oxide
- PMID: 34362839
- PMCID: PMC8762000
- DOI: 10.1136/thoraxjnl-2021-217325
Derivation of a prototype asthma attack risk scale centred on blood eosinophils and exhaled nitric oxide
Abstract
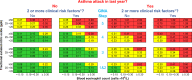
Reduction of the risk of asthma attacks is a major goal of current asthma management. We propose to derive a risk scale predicting asthma attacks based on the blood eosinophil count and exhaled nitric oxide (FeNO). Biomarker-stratified trial-level attack rates were extracted and pooled from the control arms of the Novel START, CAPTAIN, QUEST, Benralizumab Phase 2b, PATHWAY, STRATOS 1-2 and DREAM trials (n=3051). These were used to derive rate ratios and the predicted asthma attack rate for different patient groups. The resultant prototype risk scale shows potential to predict asthma attacks, which may be prevented by anti-inflammatory treatment.
Keywords: allergic lung disease; asthma; asthma epidemiology; clinical epidemiology; eosinophil biology; exhaled airway markers; pulmonary eosinophilia; respiratory measurement.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: SC: has received a non-restricted research grant from Sanofi Genzyme for investigator-initiated type 2 innovation research and speaker honoraria from Sanofi/Regeneron, AstraZeneca and GlaxoSmithKline (GSK), all outside the submitted work. AL is an employee of Sanofi Norway. MJ has received grants from the University of Oxford and National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). SR reports non-financial support from AstraZeneca and other sources of revenue from Australian Government Research Training Program and the NIHR Oxford BRC. JM has received grants from the NIHR Oxford BRC. TH has received grants from Pfizer, the University of Oxford, the Wellcome Trust, the Guardians of the Beit Fellowship and the NIHR Oxford BRC outside the submitted work. He has received personal fees from AstraZeneca, Teva and PeerVoice outside the submitted work. In the last 5 years, IP has received speaker’s honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer Ingelheim, Aerocrine AB, Almirall, Novartis, Teva, Chiesi, Sanofi/Regeneron, Menarini and GSK and payments for organising educational events from AstraZeneca, GSK, Sanofi/Regeneron and Teva. He has received honoraria for attending advisory panels with Genentech, Sanofi/Regeneron, AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Teva, Merck, Circassia, Chiesi and Knopp and payments to support FDA approval meetings from GSK. He has received sponsorship to attend international scientific meetings from Boehringer Ingelheim, GSK, AstraZeneca, Teva and Chiesi. He has received a grant from Chiesi to support a phase 2 clinical trial in Oxford. He is a copatent holder of the rights to the Leicester Cough Questionnaire and has received payments for its use in clinical trials from Merck, Bayer and Insmed. In 2014–2015, he was an expert witness for a patent dispute involving AstraZeneca and Teva.
Figures
References
-
- Global Initiative for Asthma (GINA) . Global strategy for asthma management and prevention (2021 update), 2021. Available: https://ginasthma.org/
-
- Pavord ID, Holliday M, Reddel HK, et al. . Predictive value of blood eosinophils and exhaled nitric oxide in adults with mild asthma: a prespecified subgroup analysis of an open-label, parallel-group, randomised controlled trial. Lancet Respir Med 2020;8:671–80. 10.1016/S2213-2600(20)30053-9 - DOI - PubMed
-
- Lee LA, Bailes Z, Barnes N, et al. . Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med 2021;9:69–84. 10.1016/S2213-2600(20)30389-1 - DOI - PubMed
