Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function
- PMID: 34362885
- PMCID: PMC8346468
- DOI: 10.1038/s41467-021-24956-2
Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function
Abstract
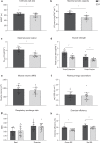
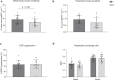
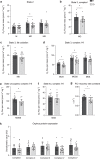
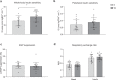
The relationship between the age-associated decline in mitochondrial function and its effect on skeletal muscle physiology and function remain unclear. In the current study, we examined to what extent physical activity contributes to the decline in mitochondrial function and muscle health during aging and compared mitochondrial function in young and older adults, with similar habitual physical activity levels. We also studied exercise-trained older adults and physically impaired older adults. Aging was associated with a decline in mitochondrial capacity, exercise capacity and efficiency, gait stability, muscle function, and insulin sensitivity, even when maintaining an adequate daily physical activity level. Our data also suggest that a further increase in physical activity level, achieved through regular exercise training, can largely negate the effects of aging. Finally, mitochondrial capacity correlated with exercise efficiency and insulin sensitivity. Together, our data support a link between mitochondrial function and age-associated deterioration of skeletal muscle.
© 2021. The Author(s).
Conflict of interest statement
J.V.d.B and M.C.E.B are affiliated with Danone Nutricia Research and Friesland-Campina, respectively. Friesland-Campina and Danone Nutricia Research are sponsors of the TiFN program and partly financed the project. The other authors declare no competing interests.
Figures


References
-
- Rodrigues, R., Huber, M. & Lamura, G. Facts and Figures on Healthy Ageing and Long-term Care. Europe and North America, Occasional Reports Series 8. Vienna: European Centre (European Centre for Social Welfare Policy and Research, 2012).

