Purple sulfur bacteria fix N2 via molybdenum-nitrogenase in a low molybdenum Proterozoic ocean analogue
- PMID: 34362886
- PMCID: PMC8346585
- DOI: 10.1038/s41467-021-25000-z
Purple sulfur bacteria fix N2 via molybdenum-nitrogenase in a low molybdenum Proterozoic ocean analogue
Abstract
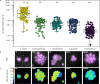
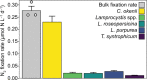
Biological N2 fixation was key to the expansion of life on early Earth. The N2-fixing microorganisms and the nitrogenase type used in the Proterozoic are unknown, although it has been proposed that the canonical molybdenum-nitrogenase was not used due to low molybdenum availability. We investigate N2 fixation in Lake Cadagno, an analogue system to the sulfidic Proterozoic continental margins, using a combination of biogeochemical, molecular and single cell techniques. In Lake Cadagno, purple sulfur bacteria (PSB) are responsible for high N2 fixation rates, to our knowledge providing the first direct evidence for PSB in situ N2 fixation. Surprisingly, no alternative nitrogenases are detectable, and N2 fixation is exclusively catalyzed by molybdenum-nitrogenase. Our results show that molybdenum-nitrogenase is functional at low molybdenum conditions in situ and that in contrast to previous beliefs, PSB may have driven N2 fixation in the Proterozoic ocean.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Stüeken EE, Kipp MA, Koehler MC, Buick R. The evolution of Earth’s biogeochemical nitrogen cycle. Earth-Sci. Rev. 2016;160:220–239. doi: 10.1016/j.earscirev.2016.07.007. - DOI
-
- Fischer WW, Hemp J, Johnson JE. Evolution of oxygenic photosynthesis. Annu. Rev. Earth Planet. Sci. 2016;44:647–683. doi: 10.1146/annurev-earth-060313-054810. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

