Responsive core-shell DNA particles trigger lipid-membrane disruption and bacteria entrapment
- PMID: 34362911
- PMCID: PMC8346484
- DOI: 10.1038/s41467-021-24989-7
Responsive core-shell DNA particles trigger lipid-membrane disruption and bacteria entrapment
Abstract
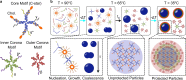
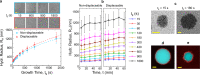
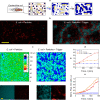
Biology has evolved a variety of agents capable of permeabilizing and disrupting lipid membranes, from amyloid aggregates, to antimicrobial peptides, to venom compounds. While often associated with disease or toxicity, these agents are also central to many biosensing and therapeutic technologies. Here, we introduce a class of synthetic, DNA-based particles capable of disrupting lipid membranes. The particles have finely programmable size, and self-assemble from all-DNA and cholesterol-DNA nanostructures, the latter forming a membrane-adhesive core and the former a protective hydrophilic corona. We show that the corona can be selectively displaced with a molecular cue, exposing the 'sticky' core. Unprotected particles adhere to synthetic lipid vesicles, which in turn enhances membrane permeability and leads to vesicle collapse. Furthermore, particle-particle coalescence leads to the formation of gel-like DNA aggregates that envelop surviving vesicles. This response is reminiscent of pathogen immobilisation through immune cells secretion of DNA networks, as we demonstrate by trapping E. coli bacteria.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Alberts, B. et al. Molecular Biology of the Cell, Parts 2 and 4 6th edn (Garland Science, 2014).
-
- Wardhan, R. & Mudgal, P. Textbook of Membrane Biology 1st edn (Springer, 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

