Equine pituitary pars intermedia dysfunction: a spontaneous model of synucleinopathy
- PMID: 34362943
- PMCID: PMC8346493
- DOI: 10.1038/s41598-021-95396-7
Equine pituitary pars intermedia dysfunction: a spontaneous model of synucleinopathy
Abstract
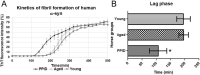
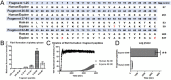
Equine pituitary pars intermedia dysfunction (PPID) is a common endocrine disease of aged horses that shows a similar pathophysiology as Parkinson's Disease (PD) with increased levels of α-synuclein (α-syn). While α-syn is thought to play a pathogenic role in horses with PPID, it is unclear if α-syn is also misfolded in the pars intermedia and could similarly promote self-aggregation and propagation. Consequently, α-syn was isolated from the pars intermedia from groups of healthy young and aged horses, and aged PPID-afflicted horses. Seeding experiments confirmed the prion-like properties of α-syn isolated from PPID-afflicted horses. Next, detection of α-syn fibrils in pars intermedia via transmission electron microscopy (TEM) was exclusive to PPID-afflicted horses. A bank of fragment peptides was designed to further characterize equine α-syn misfolding. Region 62-87 of equine and human α-syn peptides was found to be most prone to aggregation according to Tango bioinformatic program and kinetics of aggregation via a thioflavin T fluorescence assay. In both species, fragment peptide 62-87 is capable of generating mature fibrils as demonstrated by TEM. The combined animal, bioinformatic, and biophysical studies provide evidence that equine α-syn is misfolded in PPID horses.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

