Interaction of 4'-methylflavonoids with biological membranes, liposomes, and human albumin
- PMID: 34362978
- PMCID: PMC8346624
- DOI: 10.1038/s41598-021-95430-8
Interaction of 4'-methylflavonoids with biological membranes, liposomes, and human albumin
Abstract
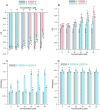
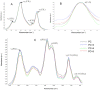
The aim of the study was to compare the impact of three synthesized chemical compounds from a group of methylated flavonoids, i.e. 2'-hydroxy-4-methylchalcone (3), 4'-methylflavanone (4), and 4'-methylflavone (5), on a red blood cell membranes (RBCMs), phosphatidylcholine model membranes (PC), and human serum albumin (HSA) in order to investigate their structure-activity relationships. In the first stage of the study, it was proved that all of the compounds tested do not cause hemolysis of red blood cells and, therefore, do not have a toxic effect. In biophysical studies, it was shown that flavonoids have an impact on the hydrophilic and hydrophobic regions of membranes (both RBCMs and PC) causing an increase in packing order of lipid heads and a decrease in fluidity, respectively. Whereas, on the one hand, the magnitude of these changes depends on the type of the compound tested, on the other hand, it also depends on the type of membrane. 4'-Methylflavanone and 4'-methylflavone are located mainly in the hydrophilic part of lipid membranes, while 2'-hydroxy-4-methylchalcone has a greater impact on the hydrophobic area. A fluorescence quenching study proved that compounds (3), (4) and (5) bind with HSA in a process of static quenching. The binding process is spontaneous whereas hydrogen bonding interactions and van der Waals forces play a major role in the interaction between the compounds and HSA.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

