Twelve-Month results from multicenter, open-label, randomized controlled clinical trial comparing differential target multiplexed spinal cord stimulation and traditional spinal cord stimulation in subjects with chronic intractable back pain and leg pain
- PMID: 34363307
- PMCID: PMC9290817
- DOI: 10.1111/papr.13066
Twelve-Month results from multicenter, open-label, randomized controlled clinical trial comparing differential target multiplexed spinal cord stimulation and traditional spinal cord stimulation in subjects with chronic intractable back pain and leg pain
Abstract
Background: Spinal cord stimulation (SCS) is a well-established treatment for chronic intractable pain of the trunk and/or limbs; however, low back pain (LBP) is difficult to treat using traditional SCS. Differential Target Multiplexed spinal cord stimulation (DTM SCS) is an advanced approach inspired from animal studies demonstrating improved pain-related behavior and pain-relevant biological processes.
Objective: The purpose of this study was to compare the effectiveness of DTM SCS and traditional SCS in treating chronic LBP and leg pain (LP).
Methods: This prospective, postmarket randomized controlled trial compared DTM SCS to traditional SCS in patients with chronic LBP and LP. Primary end point was LBP responder rate (percentage of subjects with ≥ 50% relief) at 3 months. Noninferiority and superiority were assessed. Other outcomes included mean change in back and leg pain, responder rates, disability, global health, satisfaction, and safety profile throughout the 12-month follow-up.
Results: One hundred twenty-eight subjects were randomized across 12 centers (67 DTM SCS and 61 traditional SCS). Of the 94 patients implanted, 46 subjects in each group completed the 3-month assessment. LBP responder rate of 80.1% with DTM SCS was superior to 51.2% with traditional SCS (p = 0.0010). Mean LBP reduction (5.36 cm) with DTM SCS was greater than reduction (3.37 cm) with traditional SCS (p < 0.0001). These results were sustained at 6 months and 12 months. Safety profiles were similar between treatment groups.
Conclusion: Superiority of DTM SCS compared with traditional SCS for chronic LBP was demonstrated. Clinical improvements provided by DTM SCS were sustained over 12 months and are expected to significantly impact the management of chronic LBP.
Keywords: back pain; differential target multiplexed; randomized controlled trial; spinal cord stimulation.
© 2021 The Authors. Pain Practice published by Wiley Periodicals LLC on behalf of World Institute of Pain.
Conflict of interest statement
J.C. reports consulting fees from PillNurse; honoraria or payment for lectures/speaker bureau/educational events from Abbot, Biotronik, Boston Scientific, Medtronic, and Saluda; medical advisory board membership in Mainstay Corneloc, and PillNurse; treasurer and executive board member of ASPN, president of AZSIPP; and stock/stock options in Cornerloc and Mainstay. M.F. reports research grants to institution from Abbott, Biotronik, Boston Scientific, Medtronic, Nalu Medical, SGX Medical, and Thermaquil; consulting fees from Abbott, Biotronik, Medtronic, and Nevro; faculty appointment with Abbott and Medtronic; director‐at‐large of NANS; and stock/stock options at Celeri Health, and Thermaquil. R.J. reports honoraria or payment for speaker bureau from Medtronic. P.K. reports honoraria or payment for lectures/speaker bureau/educational events from Biotronik and Medtronic. V.M. reports consulting fees from Aurora Spine and Medtronic; and stock/stock options from Aurora Spine. D.P. reports consulting fees from Avanos, Boston Scientific, Esteve, Heron, Medtronic, and Nevro; and other financial or nonfinancial interests from Abbott, Avanos, Medtronic, Nevro, and Stimgenics. M.S. reports being secretary of ASIPP. R.V. reports research grant from Medtronic, consulting fees from Stimgenics (CEO) and SGX Medical (CEO); honoraria or payment for speaker bureau from Medtronic; patents granted/pending assigned to Medtronic; medical advisory board membership in Medtronic; past director‐at‐large of NANS; stock/stock options at SGX Medical. R.B., A.C., H.C., J.F., C.M., and B.S. report no competing interests.
Figures

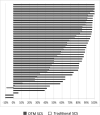


References
-
- Simon LS. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. J Pain Palliat Care Pharmacother. 2012;26(2):197–8.
-
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet‐based survey. J Pain. 2010;11(11):1230–9. - PubMed
-
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. - PMC - PubMed
-
- Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the neuromodulation appropriateness consensus committee. Neuromodulation. 2014;17(6):515–50. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

