STOP 301: A Phase 3, open-label study of safety, tolerability, and exploratory efficacy of INP104, Precision Olfactory Delivery (POD® ) of dihydroergotamine mesylate, over 24/52 weeks in acute treatment of migraine attacks in adult patients
- PMID: 34363701
- PMCID: PMC9292844
- DOI: 10.1111/head.14184
STOP 301: A Phase 3, open-label study of safety, tolerability, and exploratory efficacy of INP104, Precision Olfactory Delivery (POD® ) of dihydroergotamine mesylate, over 24/52 weeks in acute treatment of migraine attacks in adult patients
Abstract
Objective: To report the safety, tolerability, exploratory efficacy, and patient acceptability of INP104 for the acute treatment of migraine from the Phase 3 STOP 301 trial.
Background: Dihydroergotamine (DHE) has long been used to treat migraine, but intravenous administration is invasive, frequently associated with adverse events (AEs), and not suitable for at-home administration. INP104 is an investigational drug device that delivers DHE mesylate to the upper nasal space using a Precision Olfactory Delivery technology and was developed to overcome the shortcomings of available DHE products.
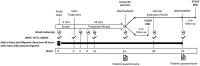
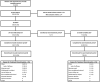
Methods: STOP 301 was an open-label, 24-week safety study, with a 28-week extension period. After a 28-day screening period where patients used their "best usual care" to treat migraine attacks, patients were given INP104 (1.45 mg) to self-administer nasally with self-recognized attacks. The primary objective of this study was to assess safety and tolerability, with a specific focus on nasal mucosa and olfactory function. Exploratory objectives included efficacy assessments of migraine measures and a patient acceptability questionnaire.
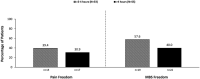
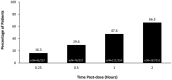
Results: A total of 360 patients entered the 24-week treatment period, with 354 patients dosing at least once. INP104-related treatment-emergent AEs were reported by 36.7% (130/354) of patients, and 6.8% (24/354) discontinued treatment due to AEs over 24 weeks. No new safety signals were observed following delivery to the upper nasal space. Pain freedom, the most bothersome symptom freedom, and pain relief at 2 h post-INP104 were self-reported by 38.0% (126/332), 52.1% (173/332), and 66.3% (167/252) of patients, respectively. A low recurrence rate at 24 and 48 h was observed (7.1% [9/126] and 14.3% [18/126], respectively). Most patients found INP104 easy to use and preferred it over their current therapy.
Conclusions: INP104 has the potential to deliver rapid symptom relief, without injection, that is well tolerated and suitable for outpatient use. Results suggest INP104 may be a promising treatment for patients with migraine.
Keywords: Precision Olfactory Delivery; dihydroergotamine; efficacy; migraine; safety/tolerability; upper nasal space.
© 2021 The Authors. Headache: The Journal of Head and Face Pain published by Wiley Periodicals LLC on behalf of American Headache Society.
Conflict of interest statement
Timothy R. Smith has supported clinical trial research for Lundbeck, Amgen, Allergan/AbbVie Inc., Biohaven Pharmaceuticals, Boehringer Ingelheim, Charleston Laboratories, Inc., electroCore, Inc., Impel NeuroPharma, Eli Lilly and Company, Pfizer Inc., Novartis, Novo Nordisk, Satsuma Pharmaceuticals, Inc., Teva Pharmaceutical Industries Ltd., Theranica Bio‐Electronics Ltd., and Vorso Corp. He has participated in speaker bureaus for Amgen, Allergan/AbbVie Inc., Biohaven Pharmaceuticals, and Eli Lilly and Company and is an advisor/consultant for Lundbeck, Amgen, Allergan/AbbVie Inc., Biohaven Pharmaceuticals, Impel NeuroPharma, Eli Lilly and Company, Theranica Bio‐Electronics Ltd., and Vorso Corp. He is also a stockholder in the UnitedHealth Group and is an officer and Board of Directors member (unpaid volunteer work for a nonprofit organization) for the National Headache Foundation. Paul Winner is a consultant for Allergan/AbbVie Inc., Amgen, Lundbeck, Novartis, and Teva Pharmaceutical Industries Ltd. He is also a speaker for Allergan/AbbVie Inc., Amgen, Eli Lilly and Company, Lundbeck, Novartis, and Teva Pharmaceutical Industries Ltd. and is involved in research with Allergan/AbbVie Inc., Amgen, Avanir Pharmaceuticals, A&Z Pharmaceutical Inc., Biogen, Impel NeuroPharma, Genentech, Eli Lilly and Company, Lundbeck, Novartis, Samus Therapeutics Inc., Supernus Pharmaceuticals Inc., and Teva Pharmaceutical Industries Ltd. Sheena K. Aurora, Stephen B. Shrewsbury, Jasna Hocevar‐Trnka, and Maria Jeleva are full‐time employees of, and stockholders in, Impel NeuroPharma. Maria Jeleva is also an options holder in Impel NeuroPharma. Stephen B. Shrewsbury is an officer of Impel NeuroPharma.
Figures


References
-
- Shrewsbury SB, Cook RO, Taylor G, Edwards C, Ramadan NM. Safety and pharmacokinetics of dihydroergotamine mesylate administered via a novel (TempoTM) inhaler. Headache. 2008;48:355‐367. - PubMed
-
- Baron EP, Tepper SJ. Orally inhaled dihydroergotamine: reviving and improving a classic. Future Neurol. 2011;6:327‐333.
-
- Shrewsbury SB, Jeleva M, Satterly KH, Lickliter J, Hoekman J. STOP 101: a phase 1, randomized, open‐label, comparative bioavailability study of INP104, dihydroergotamine mesylate (DHE) administered intranasally by a I123 Precision Olfactory Delivery (POD) device, in healthy adult subjects. Headache. 2019;59:394‐409. - PubMed
-
- Silberstein SD, Kori SH. Dihydroergotamine: a review of formulation approaches for the acute treatment of migraine. CNS Drugs. 2013;27:385‐394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

