Human TBK1 deficiency leads to autoinflammation driven by TNF-induced cell death
- PMID: 34363755
- PMCID: PMC8380741
- DOI: 10.1016/j.cell.2021.07.026
Human TBK1 deficiency leads to autoinflammation driven by TNF-induced cell death
Abstract
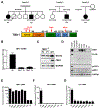
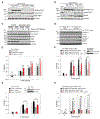
TANK binding kinase 1 (TBK1) regulates IFN-I, NF-κB, and TNF-induced RIPK1-dependent cell death (RCD). In mice, biallelic loss of TBK1 is embryonically lethal. We discovered four humans, ages 32, 26, 7, and 8 from three unrelated consanguineous families with homozygous loss-of-function mutations in TBK1. All four patients suffer from chronic and systemic autoinflammation, but not severe viral infections. We demonstrate that TBK1 loss results in hypomorphic but sufficient IFN-I induction via RIG-I/MDA5, while the system retains near intact IL-6 induction through NF-κB. Autoinflammation is driven by TNF-induced RCD as patient-derived fibroblasts experienced higher rates of necroptosis in vitro, and CC3 was elevated in peripheral blood ex vivo. Treatment with anti-TNF dampened the baseline circulating inflammatory profile and ameliorated the clinical condition in vivo. These findings highlight the plasticity of the IFN-I response and underscore a cardinal role for TBK1 in the regulation of RCD.
Keywords: IKKE; IRF3; RIPK1; TBK1 deficiency; TNF alpha; autoinflammation; interferon type I; viral susceptibility.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.B. is the founder of Lab11 Therapeutics.
Figures




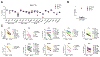
References
-
- Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen D, Thibault J, et al. (2013). From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinforma 43, 11.10.1–11.10.33. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

